Indice del volumen
Volume index
Comité Editorial
Editorial Board
Comité Científico
Scientific Committee
L-CARNITINE-INDUCED MODULATION OF PLASMA FATTY ACIDS METABOLISM IN HYPERLIPIDEMIC RABBITS
*Maritza F. Diaz Gómez PhD1, Julio A. Urbina PhD2, Flor López MSc2,
Frank Hernández Rosales PhD1
1Ozone Research Center, National Center for Scientific Research.
Havana, Cuba
2Venezuelan Institute for Scientific Research. Caracas. Venezuela
maritza.diaz @ cnic.edu.cu
Rev Electron Biomed / Electron J Biomed 2006;1:33-41
Comment of the reviewer Angel San Miguel, MD. PhD. Servicio de Análisis Clínicos. Hospital Universitario Rio Hortega. Valladolid. España
Comment of the reviewer Prof. Pilar Muñiz PhD. Área de Bioquímica y Biología Molecular. Facultad de Ciencias. Universidad de Burgos. Burgos. Spain
RESUMEN:
El presente estudio tiene como objetivo investigar si el efecto hipocolesterolémico de la suplementación L-carnitina está relacionado con el metabolismo de los ácidos grasos de las lipoproteínas. Los cambios en la composición de ácidos grasos y el contenido de colesterol fueron medidos en las lipoproteínas de seis grupos diferentes de conejos, grupo 1, dieta estándar, grupo 2, dieta estándar más L-carnitina 80 mg/kg, grupo 3, dieta estándar más 0,5 % de colesterol, grupo 4, dieta estándar y 0,5 % de colesterol más L-carnitina 80 mg/kg durante 126 días. Los grupos 5 y 6 fueron alimentados con la misma dieta del grupo 4 en un período previo de 126 días, y después de este tiempo, el grupo 5 fue alimentado con una dieta igual al grupo 1 y el grupo 6 fue alimentado con una dieta igual al grupo 2 durante 65 días.
Sin embargo, la progresión de la hipercolesterolemia fue reducida un 50 % por la administración de la L-carnitina en aquellos animales alimentados con dieta de colesterol. Cambios en la composición de ácidos grasos en los esteres de colesterol de las lipoproteínas fueron encontrados en todos los grupos de animales alimentados con L-carnitina. Durante el período de alimentación estándar, la relación de ácidos grasos saturados/insaturados fue disminuida en la LDL e incrementada en las partículas de HDL y VLDL. En el período de progresión de la hiperlipidemia la relación ácidos grasos saturados/insaturados fue ligeramente incrementada en la HDL y en la VLDL+LDL fue disminuida. En el período de regresión de la hiperlipidemia los niveles de colesterol plasmáticos fueron reducidos en un 33 % en el grupo; y la relación saturados/insaturados tuvo el mismo incremento que el observado en el período de progresión de las partículas HDL y VLDL+HDL. Además, se encontró en el grupo 6 una marcada reducción del 75 % de la placa aterosclerótica de la aorta. Se concluye que la L-carnitina, contribuye al mejoramiento del metabolismo de las lipoproteínas.
Palabras clave: L-carnitina; lipoproteinas, colesterol, conejos, ácidos grasos.
ABSTRACT
The present study was designed to examine whether the hipocholesterolemic effect of L-carnitine supplementation is related with lipoprotein fatty acid metabolism. Fatty acid compositional and cholesterol content changes were measured in lipoproteins of six different groups of rabbits. Group 1, rabbits fed a standard diet; group 2, rabbits fed standard diet plus L-carnitine 80 mg/kg bw; group 3, rabbits fed a 0.5 % cholesterol diet; group 4, rabbits fed a 0.5 % cholesterol diet plus L-carnitine 80 mg/kg b.w. These four groups were fed their diets during 126 days. Group 5 and 6 were fed the same diet as group 4 in a previous period of 126 days, and after this time, group 5 was fed the same diet as group 1, and group 6 fed the same diet as group 2, during a second period of 65 days.
However, the progression of hypercholesterolemia was reduced 50 % by L-carnitine administration in those animals fed cholesterol diet. Fatty acid compositional changes in lipoprotein-cholesteryl esters were found in all groups of animals supplemented with L-carnitine. During the standard-fed period the saturated and unsaturated fatty acid ratio was increased in VLDL and HDL particles whereas was decreased in LDL. In the hyperlipidemia progression period the saturated to unsaturated fatty acid ratio in HDL fraction was slightly enhanced and in the VLDL+LDL modified particle was diminished. In the hyperlipidemia regression period, plasma cholesterol level was additionally reduced in a 33 % in the group 6; and the saturated to unsaturated fatty ratio had the same behaviour from that observed in the progression period for HDL and VLDL+LDL particles. A remarkable reduction (75%) of aorta atherosclerotic plaques in the group 6 was found. From these results we concluded that L-carnitine, in this experimental model, induces an improved lipoprotein metabolism.
Key Words: L-carnitine; lipoproteins, cholesterol, rabbits, fatty acids.
INTRODUCTION
L-carnitine (ß-hyydroxy-g-trimethylamino butyrate) has been described as a conditionally essential nutrient for human1 and animals2. About 75 % of the carnitine source for the body stores comes from the diet. In man the liver and the kidney synthesize the remaining 25 % from the immediate precursor gamma butyrobetaine3, 4.
L-carnitine is a quaternary amine which has different biological roles including: mitochondrial long-chain fatty acid oxidation, activation of aerobic glycolisis, enhancement of respiratory chain function, buffering of the mitochondrial acyl CoA/CoA couple, scavenger system for acyl groups peroxisomal fatty acid oxidation, branched amino acid metabolism, membrane stabilization, and donor of acetyl groups for biosynthesis5. Carnitine in blood is much less concentrated than in tissues. Consequently carnitine, either introduced in the diet or synthesizes de novo in the liver and kidney, must be actively concentrated from the blood into fatty acid metabolizing organs6.
In previous experiments it has been demonstrated that L-carnitine administration is capable of reducing hyperlipidemia after fat diet feeding7, 8. Supplementation of L-carnitine derivatives to hyperlipidemic rabbits induces a marked lowering of plasma triglycerides, very low density lipoproteins (VLDL) and intermediate density lipoproteins (IDL) triglycerides, while plasma cholesterol is slightly and transiently reduced along with a reduction of aortic plaque thickness and extend9. The administration of L-carnitine in patients with type IV hyperlipoproteinemia increases carnitine and decreases triglycerides plasma levels10-13. Changes in the VLDL transport and not in lipoprotein metabolism has been proposed as the main mechanisms for lipid-lowering effect of L-carnitine in the hyperlipidemic rabbits14. However, other authors have stated that L-carnitine and its derivatives can stimulate the ß-oxidation of fatty acids that finally results in a strong control over the plasma lipid resulting in a decrease in the aortic plaque of this animal model9. In a previous paper we have suggested that the effect of L-carnitine in this experimental model could be associated with increased systemic breakdown of cholesteryl esters, a probable increase in reverse cholesterol transport, and the stabilization of a phospholipid-based structure of the abnormal VLDL + LDL particles15.
To elucidate whether lowering-lipid effect of L-carnitine is related with lipoprotein fatty acids metabolism, we examine the effect of L-carnitine treatment on fatty acid change in plasma lipoproteins during rabbit hyperlipidemia progression and regression periods.
MATERIALS AND METHODS
Animals and diets
Male New Zealand rabbits (2.5-3 kg.) were used in all experiments. Rabbits were fed with 100 g daily of a standard rabbit chow (Alimentos Protinal C.A., Valencia, Venezuela) with a partial composition of 19 % of crude protein (minimal), 2% of crude fat (minimal), 10% of crude staple (maximum) and 40% of free nitrogen extracts (minimal), and water "ad libitum". The cholesterol diet consisted of the same chow supplemented with 0.5% Cholesterol (Sigma Company, 95% of purity), dissolved in 5% of corn oil containing 15% of saturated fatty acids and 85 % of unsaturated fatty acids approximately, (Mazeite, Refinadora de Maíz Venezolana C.A., Aragua, Venezuela); by kilogram of chow. The L-carnitine (Laboratory Elmor S.A., Caracas, Venezuela) was supplied slowly by oral route through the use of a stainless steel cannula after overnight fasting. Sodium penthothal (Laboratorios Abbot, Caracas, Venezuela), was employed intravenously to anesthetize animals. The experimental protocols used in the present study conformed to accepted standards; define by the Bioethics Commission of the Venezuelan Institute for Scientific Research.
After an initial adaptation period, rabbits were randomized and separated into six groups: group 1, six rabbits fed a normal diet; group 2, six rabbits fed a normal diet plus L-carnitine in a daily dose of 80 mg/kg of corporal weight; group 3, six rabbits fed the cholesterol diet; group 4, six rabbits fed the cholesterol diet plus L-carnitine (80 mg/kg); group 5, six rabbits fed the normal diet during 65 days post 126 days cholesterol diet administration; group 6, six rabbits fed the normal diet plus L-carnitine (80 mg/kg) during 65 days post 126 days cholesterol diet administration.
Lipoprotein isolation
Blood samples were taken from marginal ear vein at day 0, after 126 days (groups 1 to 4) and at 191 days (groups 5 and 6). Blood cells were removed by centrifugation at 2 000 g for 20 min and plasma from animals of each group was pooled. The lipoproteins of each pool of plasma were isolated sequentially by differential ultracentrifugation at 110 000 g at 20°C in a Beckman rotor 60-Ti. The procedure for obtaining plasma lipoproteins has been previously described in detail16. The isolated fractions were dialyzed exhaustively against a buffer containing 0.2 M monobasic sodium phosphate, 0.2 M dibasic sodium phosphate and 0.16 sodium chloride, pH 7.4. The lipoproteins were stored al 2°C until used. The lipoprotein purity was checked by agarose gel electrophoresis.
Lipid analysis
Cholesterol and its esters, after separation by thin-layer chromatography, were measured by the procedure of Bowman and Wollf17.
Percentage of damaged area in aorta
After sacrificing the animals, the aorta extract was proceeding in its descendent segment up to the iliac artery bifurcation. The arteries were put into a preserving solution, which contained: Tris 5 mM, CLNA 0.15 M, sodium EDTA 0.5 mM, Thimerosal 0.1%, PMSF (p-methyl sulphonyl fluoride) 0.2 mM, E-aminocaproic acid 10 mM and copper sulphate 10 mM. Tunica adventitia was eliminated dissecting the tissue with scissors, and the arteries were put into the preserving solution at 4°C up to the moment of the analysis. All samples were randomly distributed for their subsequent analysis of the percentage of damage area, by lipids staining technique by the Willis method18.
Gas-liquid Chromatography
The methyl esters of fatty acids of the different fractions were prepared by direct transesterification19. The cholesteryl esters were separated by thin-layer chromatography20. Methylation was performed with 3 vol. of 6% methanolic sulfonic acid (v/v) in stoppered tubes at 85 0C for 90 min; the phases were separated by addition of 3 vol. of distilled water. The methyl esters were extracted three times with 2 vol. of chloroform. The chloroform was collected, evaporated under N2, and the residue was redissolved in 50 mL of chloroform.
The fatty acids were chromatographed as methyl esters on a 180 cm fused silica column with an internal diameter of 2 mm. The column packed with 10% SILAR 10 C coated on 30-200 mesh Diatoport S. The analyses were performed on Varian 3 700 gas chromatograph equipped with a flame ionization detector and a digital Perkin Elmer integrator. Helium was used as carrier gas (1.9 mL/min at 80 0C) and nitrogen as make-up gas. The temperature was programmed from 145 0C to 245 0C at 4 0C/min. External fatty acid standards (Sigma-Aldrich Chemical Company) were used to identify components.
Statistical analysis
All data are presented as means ± standard deviations. Where applicable, Wilcoxon or Mann Whitney tests were used to determine statistical significance at p < 0.0521.
RESULTS
Normal and hyperlipidemia progression period
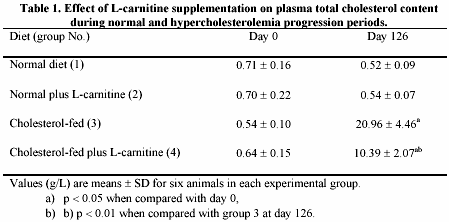
In table 1, the total plasma levels of cholesterol after L-carnitine administration to normal and cholesterol-fed rabbits are shown. Two relevant aspects are observed in this table. One is that L-carnitine had no significant effect on plasma cholesterol levels of animals fed a normal diet (group 2 vs. 1). The other one is a decreased pattern in cholesterol plasma levels (p < 0.01) from animals fed a cholesterol diet plus L-carnitine (group 4), when compared with those animals fed a cholesterol diet without L-carnitine supplementation (group 3).
The changes in fatty acid composition from cholesteryl esters of the different lipoprotein particles are presented in table 2.
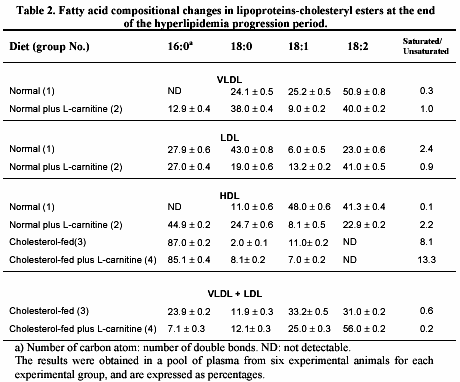
The C16 and C18 fatty acids constituted more than 70% of total fatty acids, the rest being a variable mixture of long-chain polyunsaturated acids. On VLDL particle, L-carnitine in normal fed animals induced an important increase in the levels of the saturated C16 and C18 and a decrease in the level of monounsaturated C18 (group 2 vs. 1); all these changes leading to 3 times increase in the saturated to unsaturated ratio. In contrast, L-carnitine administration produces a marked reduction of this ratio in LDL particle from the same animals (group 2 vs 1), due to reduction in the saturated C18 content and a rise in the diunsaturated C18 content. Fatty acid compositional changes mediated by L-carnitine in cholesteryl esters of HDL were observed in both normal and cholesterol-fed animals as we have previously reported (15). This substance led to a notable growth in the saturated C16 and C18, and an important decline in mono/diunsaturated C18 levels, in HDL fraction from normal-fed plus L-carnitine animals (group 2 vs. 1), given a prominent increment in the saturated to unsaturated ratio. In cholesterol-fed rabbits, the largest fraction of cholesterol and triglycerides of exogenous or endogenous origin is present in abnormal VLDL particle with electrophoretic mobility (ß-VLDL)22, which is named by other as VLDL + LDL particle (16). In our study, the fatty acid composition in cholesteryl esters of VLDL + LDL particle changes in favor of a decay in the content of saturated C16 and an enhancement in the level of diunsaturated C18 leading to a 3-fold reduction of the saturated to unsaturated ratio of those animals supplemented with L-carnitine (group 4 vs 3).
Hyperlipidemia regression period
L-carnitine-induced effects on plasma cholesterol level during the regression period of hypercholesterolemia are presented in table 3. Hypercholesterolemic animals without administration of L-carnitine (group 5) had a significant average reduction in plasma cholesterol (-11.99 g/L). Supplemented animals with L-carnitine (group 6) had a higher significant average plasma cholesterol reduction (-15.96 g/L) than rabbits of group 5. The decrease in group 6 was highly significant (p < 0.01), when compared with group 5.
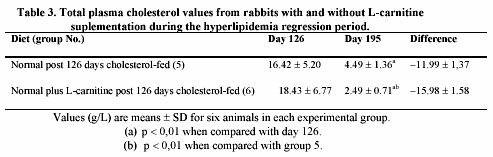
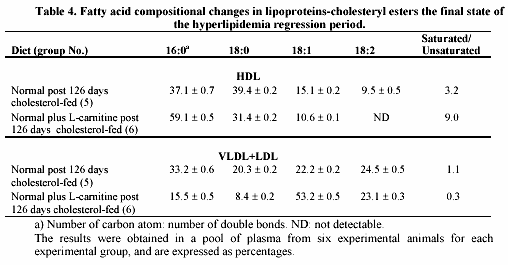
Table 4 shows some changes in fatty acids composition from cholesteryl esters of lipoproteins during the regression period. In HDL, L-carnitine induced an increased level of the saturated fatty acid C16 leading to an increase in the saturated to unsaturated ratio of approximately 3 fold (group 6 vs. 5). However, in the abnormal VLDL + LDL fraction a remarkable reduction in the saturated fatty acids C16 and C18 and an increase in monounsaturated C18 occurred when animals were supplemented with L-carnitine (group 6). In this case the saturated to unsaturated ratio decreases 4 fold.
The relationship between fatty acid compositional changes in plasma and the regression of the damaged aorta luminal area was also analyzed. Figure 1 shows that those rabbits without the administration of L-carnitine (group 5) exhibited the 83.5% of their aortas with atherosclerotic plaques at the end of the regression period (day 195), while, the animals with L-carnitine in their diet (group 6) displayed only the 21% of injury in their aortas, equivalent to a significantly reduction of 75% (p < 0.01).
DISCUSSION
Lowering lipid effect of L-carnitine has been found using several concentrations of this substance. Secombe et al. showed that administration of L-carnitine at a dose of 170 mg/kg to rabbits along with hypercholesterolemic diet, induced significant reduction in plasma VLDL-cholesterol, VLDL-triglycerides and increases the activity of hepatic acyl-CoA cholesterol acyl transferasa23. In humans, a as low dose of L-carnitine as approximately 43 mg/kg was effective in promoting lipid reduction in dyslipidemic patients24, 25. The dose of L-carnitine used in the present study (80 mg/kg) has been employed previously in humans, without adverse effects, for the treatment of different primary or secondary disturbances of the levels of this important metabolic component26, 27.
In the hyperlipidemic progression period we found that L-carnitine induced remarkable diminution in the content of saturated acids in the abnormal VLDL + LDL fraction and increased this content in HDL particle. This behavior is consistent with an increase in the fatty acid b-oxidation and/or an enhancement in the metabolism between lipoproteins. We have previously postulated that these kinds of fatty acid compositional changes could be associated with a preferential stimulation by L-carnitine of saturated fatty acid breakdown in peripheral tissues due to the specificity of the transesterifying enzymes of mitochondrial outer membranes15. If this is the case, a mayor proportion in unsaturated fatty acid would be expected in VLDL and LDL de novo biosynthesis. On the other hand, a favorable change of unsaturated fatty acids in LDL lipoprotein has been postulated to increase the metabolism of this particle28, 29. The main changes in cholesterol load are in the esterified form of cholesterol from lipoproteins of hypercholesterolemic rabbits. The decrease of cholesteryl esters in the modified fraction VLDL + LDL caused by L-carnitine suggests changes in fatty acid composition that favor the catabolism of this fraction15. The fractional catabolic rate of LDL has been observed significantly higher from the polyunsaturated fatty acid diet than from saturated regimens30. We observed a remarkable increase in the content of linoleate (C18:2) in VLDL + LDL from L-carnitine cholesterol fed rabbits (table 2, group 4 vs. 3), mimicking the pattern observed in the polyunsaturated diet studies30. In those rabbits fed normal diet, differential changes in fatty acid composition were observed in VLDL, LDL and HDL lipoprotein fractions of those animals supplemented with L-carnitine (table 2, group 2 vs. 1). Whereas saturated to unsaturated fatty acid ratio in the cholesteryl esters from LDL and HDL diminished, in contrast, this ratio rose in VLDL particle. We have not yet explanation for the differential effect of L-carnitine on VLDL fraction in normal fed rabbits. Maybe, it could be related with the fact that hepatic acyl-CoA cholesterol acyl transferase must first be stimulated by exogenous cholesterol before a stimulatory effect by L-carnitine can take place31, 32.
Evidence suggesting that supplementation of L-carnitine improved peripheral and hepatic fatty acid b-oxidation along with rise in lipoprotein metabolism can be drawn from the obtained results in the hyperlipidemia regression period. The significant reduction of cholesterol from L-carnitine fed rabbits (table 3, group 6 vs. 5) can be related with the fatty acid compositional changes in lipoprotein fractions (table 4, group 6 vs. 5). These changes were similar to those observed in the hyperlipidemia progression period. Thus, we can assume that L-carnitine effects were through the same mechanisms in both periods. However, we found a significant reduction of atherosclerotic lesions in the aortas from L-carnitine fed rabbits during the regression period (Fig. 1). This result suggests, among other things, that intensification in the cholesterol reverse transport has taken place. We have postulated (15) that increased breakdown of cholesterol esters from VLDL + LDL as a consequence of L-carnitine-induced catabolism of fatty acids, combined with transfer of cholesterol esters from VLDL + LDL to HDL, probably mediated by cholesteryl ester transfer protein33, 34, leads to increased cholesterol reverse transport and excretion35, 36. This is one of the pathways by which plaque cholesterol can be eliminated.
normal diet plus L-carnitine (group 6) during 65 days of hyperlipidemia regression period.
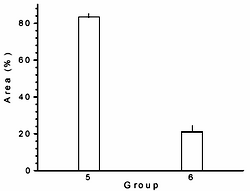
In contrast, during the progression period we have not found significant reduction of atherosclerotic plaque33. We think that during the progression period L-carnitine-induced effect on atherosclerotic plaque is overlapped due to the prolonged time of high cholesterol regimen of these animals. Therefore, regression period was a good model to see the molecular effect of this substance related with the final antiatherogenic effect. This relationship strengthens the conclusion that L-carnitine, in this experimental model, induces the peripheral and hepatic metabolism of lipoprotein fatty acids leading at the same time to an improved lipoprotein metabolism.
ACKNOWLEDGMENTS: We thank Gonzalo Visbal for technical assistance.
ECONOMIC SUPPORT: Venezuelan Institute for Scientific Research. (IVIC) supported this work. Maritza Díaz was a United Nations University fellow.
REFERENCES
-
1. Mitchell EM. Carnitine metabolism in human. Subjects. I. Normal Metabolism. Am. J. Clin. Nutr. 1978 31:293-306.
2. Rebouche CJ. Sites and regulation of Carnitine biosynthesis in mammals. Fed. Proc. 1982 41:2848-52.
3. DiDonato S. Disorders of lipid metabolism effecting skeletal muscle: Carnitine deficiency syndromes, defects in the catabolic pathway. In: Engel AG, Franzini-Armstrong C, editors, Myology, 2nd ed, New York, McGraw Hill, 1994; 1587.
4. Murray MT. The many benefits of carnitine. Am. J. Natural Med. 1996 3:6-14.
5. Arenas J, Rubio JC, Martín MA, Campos Y. Biological roles of L-carnitine in perinatal metabolism. Early Human Development 1998 53:S43-50.
6. Noseda G, Lewis B, Paoletti R. Diet and drugs in atherosclerosis. Raven Press, New York, 1980; 15.
7. Mondola P, Belfiore A, Santagelo F, Santillo M. The effect of L-carnitine on the apolipoprotein pattern of rats fed a cholesterol-rich diet. Comp. Biochem. Physiol. 1989 00B:1-5.
8. Maccari F, Arseni A, Chiodi P, Ramacci M.T, Angelucci L, Hulsmann WC. L-carnitine effect on plasma lipoproteins of hiperlipidemic fat-loaded rats. Lipids 1987 22:1005-8.
9. Spagnoli LG, Orlandi A, Marino B, Mauriello A, De Angelis C, Ramacci MT. Propionyl-L-carnitine prevents the progression of atherosclerotic lesions in aged hyperlipidemic rabbits. Atherosclerosis 1995 114:29-44.
10. Maebashi M, Kawamura N, Sato M, Imamura A, Yoshinaga K. Lipid lowering effect of carnitine in-patients with type IV hyperlipoproteinemia. Lancet 1978 2:805-8.
11. Guarnieri GF, Ranieri F, Toigo G. Lipid-lowering effect of carnitine in chronically uremic patients treated with maintenance hemodialysis. Am. J. Cli. Nutr. 1980 33:1489-92.
12. Lacour B, Di Giulio S, Chanard J. Carnitine improves lipid anomalies in haemodialysis patients. Lancet 1980 2:763-64.
13. Bellinghieri G. Carnitine and hemodialysis. Am. J. Kidney Dis. 2003 41:S116-22.
14. James L, Bhuiyan AKMJ, Foster D, Secombe D. Effect of L-carnitine treatment on Very Low Density Lipoprotein kinetics in the hyperlipemic rabbits. Clin. Biochem. 1995 28:451-458.
15. Díaz M, López F, Hernández F, Urbina JA. L-carnitine effect on chemical composition of plasma lipoproteins of rabbits fed with normal and high cholesterol diets. Lipids 2000 35:627-32.
16. Camejo J, Bosch V, Arreaza C, Mendez HC. Early changes in plasma lipoprotein structure and biosynthesis in cholesterol fed rabbits. Journal of Lipid Research 1973 14:61-8.
17. Browman RE, Wolf RC. A rapid and specific ultramicro method for total serum cholesterol. Clin. Chem. 1962 8:302-9.
18. Willis AL. Antiatherosclerosis effects of nicardipine and nifedipine in cholesterol fed rabbits. Arteriosclerosis 1985 5:250-8.
19. Lepage G, Roy CC. Direct transesterification of all classes of lipids in a one-step reaction. Journal of Lipid Research 1986 27:114-20.
20. Bitman J, Wood DL, Ruth JM. Two-stage one-dimensional thin-layer chromatographic method for separation of lipid classes. J. Liq. Chromatogr. 1981 4:1007-21.
21. Gad SC, Weil CS. Statistics for toxicologists. Principles and methods of toxicology. Raven Press, New York, 1982; p.1.
22. Mahley RW, Innerarity TL, Brown MS, Ho YK, Goldstein JL. Cholesteryl ester synthesis in macrophages: stimulation by b-very low density lipoproteins from cholesterol fed animals of several species. J. Lipid Res. 1980 21:970-76.
23. Secombe DW, James L, Hahn P, Jones E. L-carnitine treatment in hyperlipidemic rabbits. Metabolism 1987 36:1192-96.
24. Pola P, Savi L, Grilli M, Flore R, Serricchio M. Carnitine in the therapy of dyslipidemic patients. Current Ther.Res. 1980 27:208-16.
25. Stefanutti C, Vivenzio A, Cucani G. Effect of L-carnitine on plasma lipoprotein fatty acids pattern in patients with primary hiperlipoproteinemia. Clin. Ter. 1998 149:115-19.
26. Goa KL, Brogden RN. L-carnitine. A review of its characteristics and therapeutic use in cardiac ischemya and in primary and secondary deficits of carnitine, associated to fatty acid metabolism. Drugs 1987 34:1-10.
27. Pauly DF, Pepine CJ. The role of carnitine in myocardial dysfunction. Am. J. Kidney Dis. 2003 41:S35-S43.
28. Shepherd J, Packard CJ, Grundy SM, Yeshurum D, Gotto AM, Tauton OD. Effect of saturated and polyunsaturated fat diets on the chemical composition and metabolism of low density lipoproteins in man. J. Lipid. Res. 1980 21:91-9.
29. Talavera EM, Zafra MF, Gil-Villarino A, Pérez M.I, Alvarez JM, García-Pelegrin F. Changes in chemical composition and physicochemical properties of chick low and high density lipoproteins induced by supplementation of coconut oil to the diet. Biochimie, 1997 79:333-40.
30. Goodnight SH, Harris WS, Connor WE, Illingworth DR. Polyunsaturated fatty acids hyperlipidemia and thrombosis. Arterioclerosis 1982 2:87-113.
31. Bell FP. Arterial cholesterol esterification by acylCoA cholesterol acyltransferase: Its possible significance in atherogenesis and its inhibition by drugs. In: Fears R, Levy RL, Shepherd CJ, Packard JR, and Miller NE, editors, Pharmacology Control of Hyperlipidemia. Science Publisher, Barcelona, 1986; p. 409.
32. Bell FP, Vidmar TJ, Raymond TL. L-carnitine administration and withdrawal affect plasma and hepatic carnitine concentrations, plasma lipid and lipoprotein composition, and in vitro hepatic lipogenesis from labeled mevalonate and oleate in normal rabbits. J. Nutr. 1992 122:959-66.
33. Tall AR. Plasma cholesteryl ester protein. J. Lipid Res. 1993 34:1255-74.
34. Bruce C, Chavinord RA, Tall AR. Plasma lipid transfer proteins high density lipoproteins and reverse cholesterol transport. Annu. Rev. Nutr. 1998 18:297-330.
35. Fielding CJ, Fielding PE. Molecular physiology of reverse cholesterol transport. J. Lipid Res. 1995 36:211-28.
36. Díaz M, Urbina JA, López F, Hernández F. L-carnitine en la disminución de la hipercolesterolemia y la regresión de la ateromatosis. Rev. CENIC Ciencias Biológicas, 2000 31:29-34.
The aim of this work was to examine whether the hipocholesterolemic effect of L-carnitine supplementation is related with lipoprotein fatty acid metabolism. As methodology, the authors measured the fatty acid compositional and cholesterol content changes in lipoproteins of hipercholesterolemic and normal rabbits, before and after L-carnitine supplementation, by the procedure of Bowman and Wolf. (Previously, cholesterol and its ester were separated by thin-layer chromatography). As results, the authors have found fatty acid compositional changes in lipoprotein-cholesteryl ester in all groups of animals supplemented with L-carnitine. Also they have found that: (1) during the standard-fed period, the saturated and unsaturated fatty acid ratio was increased in VLDL and HDL particles whereas was decreased in LDL; (2) in the hyperlipidemia regression period, plasma cholesterol level was additionally reduced in a 33%; (3) the saturated to unsaturated fatty ratio had the same behaviour from that observed in the progression period for HDL and VLDL+LDL particles; and (4) a remarkable reduction of 75% of aorta atherosclerotic plaques in a group. From these results the authors concluded that L-carnitine induces an improved lipoprotein metabolism.
I think that both the experimental model proposed by the authors and the measures that they have carried out are accurate. Also, I believe that the aim of the work it was reached.
The authors have carried out an interesting study that suggests the potential role of L-carnitine in the lipoprotein metabolism.
In this paper the authors studied the effect of L-carnitine on fatty acid lipoproteins during rabbit hyperlipidemia progression and regression periods. The authors observed that the supplementation with L-carnitine reduced significantly the progression of hypercholesterolemia and that this can be related with the changes observed in the composition of fatty acid of lipoproteins fractions.
Finally, they describe an important reduction of aorta atherosclerotic plaques in rabbits whose diet had been supplemented with L-carnitine during the hypelipidemia regression period.
* Corresponding author:
Received: January 23, 2006.
Comment of the reviewer Angel San Miguel, MD. PhD Servcio de Análisis Clínicos. Hospital Universitario Rio Hortega. Valladolid. España
Comment of the reviewer Prof. Pilar Muñiz PhD. Área de Bioquímica y Biología Molecular. Facultad de Ciencias. Universidad de Burgos. Burgos. Spain
Dra. Maritza F. Díaz Gómez
Centro de Investigaciones del Ozono (CNIC)
Avenida 15 y calle 230, # 1313, Siboney, Playa,
Ciudad de La Habana, Cuba
E-mail: maritza.diaz @ cnic.edu.cu
Published: February 12, 2006.
