Indice del volumen Volume index
Comité Editorial Editorial Board
Comité Científico Scientific Committee
INFLUENCE OF ARYLPIPERAZINES AROMATIC
STRUCTURE OVER DIFFERENTIAL AFFINITY
FOR 5-HT1A AND 5-HT2A RECEPTORS
António Machado, Eduardo Tejera, Irene Rebelo
IBMC, Department of Biochemistry, Faculty of Pharmacy,
University of Porto. Porto, Portugal
machadobq @ gmail.com
Rev Electron Biomed / Electron J Biomed 2009;2:9-19
Comment of the reviewer Prof. Pilar Muñiz Rodríguez PhD. Titular del Área de Bioquímica y Biología Molecular de la Facultad de Ciencias de la Universidad de Burgos. España.
Comment of the reviewer Prof. Amalio Garrido Escudero PhD. Head Environmental Engineering and Toxicology Dpt. Universidad Católica S. Antonio. Guadalupe. Murcia. España.
RESUMEN:
INFLUENCIA DE LA ESTRUCTURA AROMÁTICA DE LAS ARILPIPERAZINAS EN LA AFINIDAD DIFERENCIAL CON LOS RECEPTORES 5-HT1A Y 5-HT2A
Las piperazinas son una familia de compuestos químicos muy amplia y con una gran capacidad para interactuar con diversos receptores serotonérgicos (5-HT). Debido a estas propiedades, estos compuestos tienen un importante potencial farmacológico, sin embargo muestran también algunos efectos tóxicos asociados. En la actualidad el subtipo 1A del receptor serotonérgico (5-HT1A) ha resultado ser un importante blanco para el tratamiento eficaz de la depresión y ansiedad, mientras que el subtipo 2A del receptor serotonérgico (5-HT2A) ha sido asociado con numerables efectos adversos.
En este estudio, se utilizan diversos métodos computacionales con el fin de efectuar una caracterización de los fragmentos estructurales y las propiedades químicas asociadas, responsables por la afinidad de las piperazinas para los receptores 5-HT1A Y 5-HT2A. En este trabajo, se discuten también, algunas propiedades de las estructuras aromáticas en las arilpiperazinas que son similares para los dos subtipos del receptor serotonérgico. Por otra parte se sugiere, que la substitución con calcógenos en la posición orto- y meta- así como el ligero incremento en el peso molecular son modificaciones que pueden aumentan la afinidad para el receptor 5-HT1A; mientras que las arilpiperazinas con substitución por halógenos en las mismas posiciones además de un pequeño decrecimiento en el peso molecular podrían incrementar la afinidad para el 5-HT2A receptor.
PALABRAS CLAVE: Piperazina; Receptor serotonérgico; Farmacóforos;
Afinidad; Selectividad; Diseño de fármaco
SUMMARY:
The piperazines are a large family of compounds with an enormous potential for interacting with several serotonin (5-HT) receptors. Those compounds reveal prospect for use as drugs with diverse therapeutic applications, despite the fact that they also show some toxicological effects. Actually, the subtype 1A of 5-HT (5-HT1A) receptor is responsible for efficient treatment of anxiety and depression, while subtype 2A of the 5-HT (5-HT2A) receptor is accountable for several adverse effects. In this study, we applied several computational approaches to better describe the most important chemical and substructure properties that are responsible to influence the affinity of arylpiperazines to the 5-HT1A or the 5-HT2A receptors. In the present work we discuss some properties of the arylpiperazines aromatics structures that are similar for both serotonin receptors. However, consequently, we showed that the chalcogens substitution close to the benzene ortho- and meta- position as well as a slight increment in the molecular weight showed more affinity to the 5-HT1A receptor. While arylpiperazines with halogens substitution at the same benzene position as well as a minor decrease in the molecular weight had more affinity for the 5-HT2A receptors.
KEY WORDS: Piperazine; Serotonergic receptor; Pharmacophore; Afinitty; Selectivity; Drug design.
INTRODUCTION
The interest in the role of serotonin (5-HT) and the mechanism of action of antipsychotic drugs (APDs) is the result to its direct and indirect effects on various 5-HT receptors, especially the 1A and 2A subtype serotonergic receptors (5-HT1A and 5-HT2A, respectively). Thus, both 5-HT2A antagonism and 5-HT1A agonism may be the most important of the 5-HT receptors for APD action. Postsynaptically, both 5-HT1A and 5-HT2A receptors are localised on the pyramidal neurones in the cortex, where the 5-HT1A receptor inhibits neuronal output by activation of a hyperpolarising potassium current, and the 5-HT2A facilitates output via activation of phospholipase C1,2.
This opposition between the two 5-HT receptor subtypes suggests that agonists at 5-HT1A receptors may modulate dopaminergic neurotransmission in the brain in a similar fashion to 5-HT2A receptor antagonists. The 5-HT1A receptor agonists can stimulate the release of dopamine (DA) in the prefrontal cortex as well as potentiate the effect of 2 subtype dopamine receptor (D2) blockers on DA release3. These studies suggest that 5-HT1A receptor activation is critically involved in the regulation of DA release in these two brain regions, which are involved in key cognitive function.
5-HT1A receptors are located both presynaptically and postsynaptically. The presynaptic receptors are also known as autoreceptors and are stimulated automatically upon release of serotonin. Activation of the 5-HT1A autoreceptors inhibits the release of serotonin on a global level3,4. Several studies suggest that atypical antipsychotics exert their effects on dopaminergic neurotransmission, at least in part, via activation of 5-HT1A receptors4, presumably due to concomitant potent 5-HT2A and relatively weak D2 receptor antagonism5. Cai et al. 6 suggested this may be a mechanism by which 5-HT1A receptors modulate memory and anxiety.
The use of 5-HT1A receptor agonists may substitute for 5-HT2A antagonism and achieve many of the same benefits in combination with weak D2 receptor blockade. All these studies1-7 focuses on the regulation of central 5-HT1A receptor function as an ideal target to antidepressant drugs by 5-HT1A receptor agonists underlies the therapeutic efficacy of these drugs.
The 5-HT1A receptor is present in high density in serotonergic cell body areas, in particular the dorsal and median raphe nuclei, as well as in cortical and limbic areas (e.g. frontal cortex, entorhinal cortex, hippocampus, amygdala, septum)8-10. It's also present in the hypothalamus where play important roles in the regulation of neuroendocrine function and responses to stress.
Anxiolytic or antidepressant efficacy may be due in part to compensatory changes distal to the 5-HT1A receptor receptor, such as regulation of G protein expression or reduced capacity of the receptor to activate G protein due to regulatory processes (e.g. phosphorylation) at the level of the G protein7.
The increase in serotonin neurotransmission, due to somatodendritic autoreceptor desensitization, to normo-sensitive 5-HT1A receptors in certain brain regions (e.g. hippocampus or cortex) and to sub-sensitive 5-HT1A receptors in other brain regions (e.g. amygdala or hypothalamus) underlies the therapeutic efficacy of these drugs7 (Figure 1).
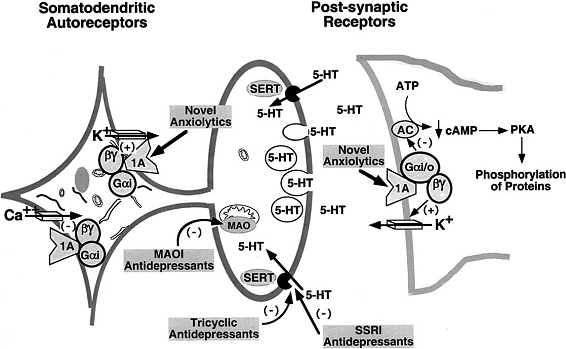
Figure 1. Anxiolytic and Antidepressant Drug Effects on Serotonergic Neurotransmission (Adaptation from Hensler et al.7).
The 5-HT1A receptor is located on serotonergic cell bodies and dendrites, functioning as the somatodendritic autoreceptor.
Several compounds are agonists at the 5-HT1A receptor, comprising anxiolytic and antidepressant activity.
By blocking the serotonin transporter (SERT) or inhibiting monoamine oxidase (MAO),
antidepressant drugs increase the synaptic concentration of the serotonin neurotransmitter (5-HT).
The piperazines (Figure 2) are an important family of compounds with vast pharmacological properties from their interactions with several 5-HT receptors, in particular, the 5-HT1A. However, one assumes that this family has the same mechanism of action and toxicity as amphetamines and ecstasy11-16. Actually, the most adverse effects are supposed to be due to the agonist interaction of the piperazines with the 5-HT2A17. Indeed, Capela et al.18-19 demonstrated, in vitro, that the overstimulation of 5-HT2A receptor is responsible for the cortical neuron's death.
Figure 2. Piperazine functional group.
Furthermore, the adverse effects on 5-HT2A receptors associated with piperazine consumption are usually schizophrenic symptoms and cerebral cortex disorders18,20, in part, due to the 5-HT2A receptors agonist interaction. On the other hand, the 5-HT1A receptors are responsible for a central modulation of affective disorders, such as anxiety and depression, revealing an enormous potential for antipsychotic drugs21, as previously referred.
Our goal consisted on identifying the chemical and molecular properties in the piperazine family that determine the selectivity and the affinity for 5-HT1A and 5-HT2A receptors. So, it is possible to improve drug design for compounds with more affinity and selectivity for the 5-HT1A receptor. For this purpose, mathematics and statistics methods were used for our analysis from the arylpiperazines in relation to the two receptor targets.
In the last decade, several Quantitative-Structure Activity Relationships (QSAR) studies were made of arylpiperazines their chemical and molecular properties relevant to the 5-HT1A and 5-HT2A receptors, as well, to pharmacophores22-25. It was shown that electro-topologic structure and substructure distances were the principal factors involved in the structure-activity correlation, although they also were related to the compounds' lipophilicity25.
The structural diversity of 5-HT2A ligands represents a challenge for pharmacophore definition, although some proposed models exist, as we already referred. Tammy et al.26 believed that the presence of a basic nitrogen group as a central point of the ligand-receptor interaction maybe questionable. Indeed, their research illustrated, at least in the piperidine family, that the basic nitrogen group substitution didn't affect ligand's afinity in relation to 5-HT2A receptors and reduced, actually, the possibility of interaction with other receptors. So, our research tried a new biochemical interaction approach that makes possible the design of new drugs and computational simulations.
MATERIALS AND METHODS
Data Set
The pharmacophore characterizations were developed from a group of molecular descriptors (1D, 2D and 3D) obtained from eDragon through a study set of a hundred and twenty four arylpiperazine derivates (Tables 1-7 in the annexes).
Computational methods
Structures of all arylpiperazine derivates were drawn on the ChemDrawn27 software package, pre-optimized by molecular mechanics using the MM2 force field. The final structure was obtained by subsequent optimization with AM1 semi empirical Hamiltonian, implemented in the MOPAC 6.0 program28.
Molecular descriptors (n=1666) were calculated for each molecular structure using eDragon software29-32 while the appropriate descriptor selection was made by means of the genetic algorithm (GA) program designed in Matlab v7.0 for this purpose. After a previous analysis of the initial descriptor set considering: a variability of more than 90% by descriptor and the elimination of the descriptors that present more than 80% of correlation between them, we obtained 572 and 573 final descriptors from eDragon for the 5-HT1A and 5-HT2A receptors, respectively.
For GA analyses33,34, the procedure was composed of 600 chromosomes chosen by a probabilistic form and the crossover was performed uniformly and with a probability of 50%. This procedure was fixed at 1 000 effective iterations. Next, the mutation procedure was carried out by changing the genes (variables) of the chromosomes with a fixed probability (50%). The crossover/mutation steps were repeated several times until they had reached a fixed stop criterion. In our model, the stop criterion was fixed at 100 000 iterations. The new chromosomes obtained after crossover/mutation procedure were evaluated with the objective function of leave-one-out internal cross-validation (Q2LOO) and was only included in the population if the Q2LOO value was higher than any of the chromosomes already considered in the initial population.
To avoid procedure complications by the structural diversity present in the piperazine's data set, we performed the subdivision of the initial number of piperazines into five subgroups by their structural similarity through cluster analysis from Moloc software35,36. For each cluster, a model was obtained by the combination of the GA and other parameters for model validation, such as the bootstrap internal cross-validation (Q2BOO) and the leave-multiple-out internal cross-validation (Q2LMO). The Q2LMO was calculated considering only a group of molecules (around 33%) for the model construction. While the Q2BOO, a more accepted internal cross-validation, was calculated by taking samples randomly and repeatedly of size N (where N is the molecules number) for the construction of the model and predicting the remaining molecules. This procedure was repeated several times (we used 5000 repetitions) and the Q2BOO represented the mean predictability coefficient. For the multiple linear regression (MLR) evaluation we used the Statistical Package37,38. Therefore, we elaborated the pharmacophore model of 5-HT1A and 5-HT2A receptors, after flexible alignment. The pharmacophore groups identification as well as the flexible alignment were performed with Moloc software36.
RESULTS AND DISCUSSION
The vast number of molecular descriptors obtained in eDragon software were simplified by eliminating the low variation and high correlated descriptors in the data set and, therefore, eliminating a large part of background noise from the essential information in our descriptors. The next step consisted of cluster analysis and, consequently, the formation of subgroups from initial piperazines set by the application of the respective method in Moloc software35 (Figure 3). That previous procedure allowed us to get five groups of piperazines with high structural similarity and made possible a better extrapolation of the mutual information shared by the flexible alignment in each group.
Figure 3 presents the initial study set (129 piperazines) division into several clusters by structural similarity shared between them. The number of clusters is proportional to structural similarity shared by the molecules in each subgroup. The selected clusters are pointed out in the figure by the numbers (1-5), which we believe to be a better equilibrium between the chemical characterization and the application domain.
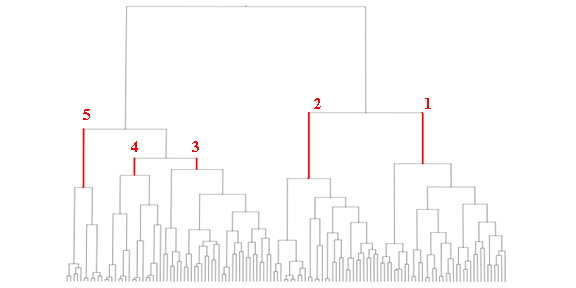
Figure 3. Dendogram obtained by the Moloc cluster analysis of the initial molecular set. The selected subgroups (1 until 5) are marked in red.
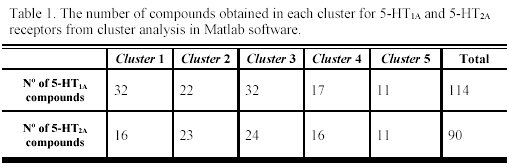
It is interesting to note that the five clusters cloud did not point up the same molecules for each receptor (as can be observed in Table 1), because not every molecule from the study set showed simultaneously a known pKi for 5-HT1A and 5-HT2A receptors. For each selected cluster a predictive model was obtained with the combination of the GA and the validation method previously referred. The obtained models and the selected descriptors are presented in the Tables 2 and 3.
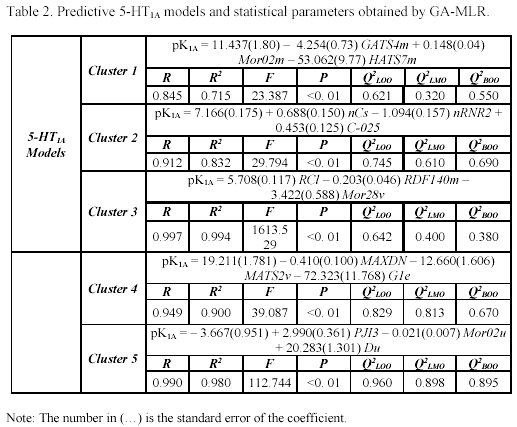
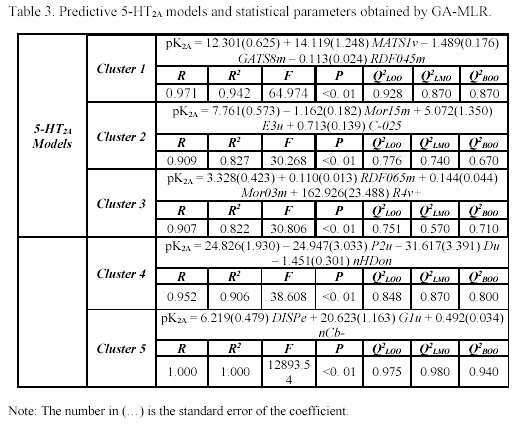
It's important to remember that the interpretation of molecular properties are extremely difficult, in particular, some classes of eDragon descriptors. Therefore, we used a correlation matrix analysis between the selected descriptors of each cluster and the easily interpretable descriptor family, such as constitutional descriptors, functional groups, atom-centred fragments and few other descriptors, to a better understanding of the structural similarity criteria applied in pharmacophore models by Moloc software35. This procedure allowed us to observe, thought an understanding way, the shared molecular information common in the interaction with both subtypes of serotonergic receptors and compare these molecular aspects with previous researches22-26.
The GA selected descriptors are strongly related to flexibility (number of rotatable bonds), molecular weight, aromatic substructure and atom type involved in the aromatic substitution; and all they represent the principal factors involved in 5-HT receptor selectivity and affinity. Besides the principal piperazine group, both receptors had affinity to aromatic groups with strong electrotopological or electronegative substituents and the presence of RCONHR bonds in the molecule, which are similar to the peptide bonds observed in several intrinsic proteins in humans. The two subtypes of serotonin receptors shared an empathy with heteroatoms' alpha carbons, such as oxygen and nitrogen elements, and aliphatic amines. On the opposition, we observed the lack of empathy with the bromine element and some functional groups, more exactly -N(CO)2 and -CX3 (where X is a certain chalcogen or halogen element).
On the one hand, the 5-HT1A receptor showed more specificity for arylpiperazines with chalcogens, such as oxygen and sulphur elements, in particular. On the other hand, the 5-HT2A receptors had more affinity for arylpiperazines with halogens, such as fluorine and chlorine elements. Besides all that, both receptors revealed a crescent affinity when those periodic elements were present in the arylpiperazine aromatic structures at ortho- and metha- positions, in particular. The effect of halogen or chalcogen elements on the arylpiperazine selectivity is probably related to the spatial arrangement of the group in the aromatic ring and its influence on the final arylpiperazine conformation, as observed by Gaillard et al.24 and López-Rodríguez et al.25.
Although molecular weight and saturation index were more or less the same in each cluster, we noted a very small tendency for the 5-HT1A receptor when those factors were slide arises. While 5-HT2A receptor had more affinity for arylpiperazines with less molecular weight and saturation index, the number of substituents in aromatic group of arylpiperazine, which allowed rotative bonds, was more daring for the 5-HT2A receptor.
In pharmacophore construction, we selected one group of molecules that demonstrate an elevated affinity index for one receptor and, simultaneously, a minor affinity index for the other receptor. So those subgroups from the study set should represent the most important intrinsic physico-chemical properties for each serotonin receptor and, therefore, facilitate our interpretation. Next, each previous subgroup was submitted to flexible alignment by a superposition algorithm from Moloc software35. This made possible the maximum superposition of molecules and their saturated bonds, in particular. So, common molecular properties were valued in pharmacophore model construction (Figure 4).
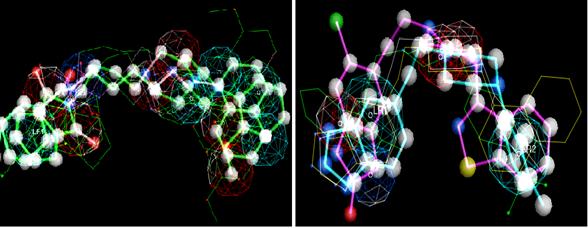
Figure 4. Illustrations of 5-HT1A and 5-HT2A pharmacophores in the left and right pictures, respectively, obtained from Moloc software.
Legend - The pictures of the spheres representing specific three-dimensional conformational characteristics, more exactly:
Dark blue sphere - Hydrogen atom donors (acid group). Light blue sphere - Aromaticity. Red sphere - Hydrogen atom acceptors (base group)
Figure 4 show a basic centre in both models, more exactly, the existence of a proton acceptor in the N4 functional group from piperazine (red sphere), while the light blue sphere demonstrated the steric effect due to aromatic substructure predominance around the N4 atom. However, the 5-HT2A model exhibited a more steric shield without losing the N4 atom's basic character. The presence of electronegativity group density, such as the carbonyl group (C=O), shared proportionately a certain basic character in the molecule and it was more visible in 5-HT1A model. Furthermore, acid groups (dark blue sphere) revealed a higher influence in the 5-HT2A receptor interaction, instead of the 5-HT1A receptor, due to their positions in relation to hydrophobic centres of arylpiperazines. These results were in accordance with Chidester et al.35. In spite of all this, the pharmacophore constituted a standard model from the common properties given by certain number of molecules and, so, was dependent on local molecular properties from some privileged structures. The N4 atom's basic character, the carbonyl group and the aromatic rings' pi ( ) density were some examples of such privileged structures present in our study set.
) density were some examples of such privileged structures present in our study set.
As we can note, both pharmacophore models share some common characteristics, such as aromatic rings and one basic group, mainly a basic nitrogen group. Actually, those structures had the predisposition to form a triangular and linear rearrangement20,26. We observed a major complexity in the 5-HT2A pharmacophore due to the existence of carbonyl group between a N4 atom (see Figure 5) and an aromatic ring in some arylpiperazines (molecules 78, 92, 128 and 129 in the annexes). These circumstances tend to modify the pharmacophore's model itself but preserving its high affinity for the receptor.
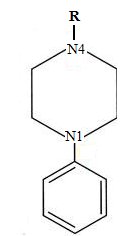
Figure 5. Representation of N4 atom present in the arylpiperazine functional group.
In the performed analysis we studied the properties of the arylpiperazines family principally those aspects related to the aromatic neighbouring. Therefore, the analysis of ligand-receptor interaction needs a further study, such as molecular docking, for a better understanding of how the analysed molecular properties are adjusted with the local environment of the actives sites in the 5-HT receptors.
CONCLUSIONS
We can conclude that arylpiperazines family share similar aromatic structure properties of interaction with 5-HT1A and 5-HT2A receptors, such as i) strong electrotopologic or electronegative substituents, ii) substructures with hydrogen donor and acceptor groups between aromatic group and N4, iii) N and O elements as substituents in benzene (Bz-N1-N4) with the capacity of establish hydrogen bonds, iv) and antipathy with Br element and specific groups (N(CO)2 and CX3, X = halogen or chalcogen).
Furthermore, there also exits diverse substructures that allowed altering the intrinsic arylpiperazine affinity to select a specific subtype of 5-HT receptor. The 5-HT1A pharmacophore model studied demonstrate that i) a basic group high accessibility, ii) an electronegative substructure near the ortho position aromatic ring and iii) and opposite lipophilic and electrostatic effects in the nitrogen substituent were extremely important for the affinity of arylpiperazines family. In particular, the presence of aromatic substructures at ortho- and metha- positions, inhibition of their rotative bonds and the attenuated increase of the saturation index and molecular weight are chemical properties that allowed the increase of arylpiperazines' affinity for the 5-HT1A receptor to the detriment of the 5-HT2A receptor.
Finally, arylpiperazines without H-donor groups or halogen atoms and with electronic density or chalcogens (e.g. O and S) close to the benzene ortho- and metha- substitution position were associated with high increment of 5-HT1A receptor affinity. Our study allows confirming previously experimental data and, more importantly, to understand the electrotopologic and three-dimensional. arylpiperzine's substructures importance in the selectivity of subtype 5-HT receptors.
However, ligand-receptor interaction properties need further investigation thought molecular docking or other computational approach.
REFERENCES
- 1. Araneda R, Andrade R. 5-Hydroxytryptamine2 and 5-hydroxytryptamine1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience 1991; 40: 399-412.
2. Martin-Ruiz R, Puig MV, Celada P, Shapiro DA, Roth BL, Mengod G, Artigas F. Control of serotonergic function in medial prefrontal cortex by serotonin-2A receptors through a glutamate-dependent mechanism. J Neurosci 2001;21: 9856-9866.
3. Meltzer HY, Li Z, Kaneda Y, Ichikawa J. Serotonin receptors: their key role in drugs to treat schizophrenia. Progress in Neuro-Psychopharmacology & Biological Psychiatry 2003; 27: 1159- 1172.
4. Millan MJ. Improving the treatment of schizophrenia: focus on serotonin 5-HT1A receptors. J Pharmacol Exp Ther 2000; 295: 853- 861.
5. Ichikawa J, Ishii H, Bonaccorso S, Fowler WL, O'Laughlin IA, Meltzer HY. 5-HT2A and D2 receptor blockade increases cortical DA release via 5-HT1A receptor activation: a possible mechanism of atypical antipsychotic-induced cortical dopamine release. J Neurochem 2001; 76: 1521-1531.
6. Cai X, Gu Z, Zhong P, Ren Y, Yan Z. Serotonin 5-HT1A receptors regulate AMPA receptor channels through inhibiting Ca2+/calmodulin-dependent kinase II in prefrontal cortical pyramidal neurons. J Biol Chem 2002; 277: 36553-36562.
7. Hensler JG. Regulation of 5-HT1A receptor function in brain following agonist or antidepressant administration. Life Sciences 2003; 72: 1665-1682.
8. Hensler JG, Kovachich GB, Frazer A. A quantitative autoradiographic study of serotonin1A receptor regulation: Effect of 5,7-dihydroxytryptamine and antidepressant treatments. Neuropsychopharmacology 1991; 4:131-44.
9. Kia HK, Miquel MC, Brisorgueil MJ, Daval G, Riad M, El Mestikawy W, Hamon M, Verge D. Immunocyotchemical localization of serotonin1A receptors in the rat central nervous system. Journal of Comparative Neurology 1996; 365: 289-305.
10. Vergé D, Daval G, Marcinkiewicz M, Patey A, El Mestikawy S, Gozlan H, Hamon M. Quantitative autoradiography of multiple 5-HT1 receptor subtypes in the brain of control or 5,7-dihydroxytryptamine-treated rats. Journal of Neuroscience 1986; 6: 3474-82.
11. Wood DM, Dargan PI, Button J, et al. Collapse, reported seizure-and an unexpected pill. Lancet 2007; 369: 1490.
12. Balmelli C, Kupferschmidt H, Rentsch K, Schneemann M. Fatal brain edema after ingestion of ecstasy and benzylpiperazine. Dtsch Med Wochenschr 2001; 126 (28-29): 809-11.
13. Gee P, Richardson S, Woltersdorf W, Moore G. Toxic effects of BZP-based herbal party pills in humans: a prospective study in Christchurch, New Zealand. N Z Med J 2005; 118 (1227): U1784.
14. Wikstrom M, Holmgren P, Ahlner J. A2 (N-benzylpiperazine) a new drug of abuse in Sweden. J Anal Toxicol 2004; 28: 67-70.
15. de Boer D, Bosman I, Hidvégi E, Manzoni C, Benkö A, dos Reys L, Maes R. Piperazine-like compounds: a new group of designer drugs-of-abuse on the European market. Forensic Sci Int 2001; 121 (1-2): 47-56.
16. Sheridan J, Butler R, Wilkins C, Russell B. Legal piperazine-containing party pills - a new trend in substance Misuse. Drug and Alcohol Review 2007; 26: 335-343.
17. Baumann MH, Clark RD, Budzynski AG, Partilla JS, Blough BE, Rothman RB. N-substituted piperazines abused by humans mimic the molecular mechanism of 3,4-methylenedioxymethamphetamine (MDMA, or 'Ecstasy'). Neuropsychopharmacology 2005; 30: 550-60.
18. Capela J, Ruscher K, Lautenschlager M, Freyer D, Dirnagl U, Gaio A, Bastos M, Meisel A, Carvalho F. Ecstasy-induced cell death in cortical neuronal cultures is serotonin 2A-receptor-dependent and potentiated under hyperthermia. Neuroscience 2006; 139: 1069-1081.
19. Capela JP, Fernandes E, Remião F, Bastos ML, Meisel A, Carvalho F. Ecstasy induces apoptosis via 5-HT2A-receptor stimulation in cortical neurons. NeuroToxicology 2007; doi:10.1016/j.neuro.2007.04. 005.
20. Rowley M, Bristow LJ, Hutson PH. Current and Novel Approaches to the Drug Treatment of Schizophrenia. Journal of Medicinal Chemistry 2001; Vol. 44: No. 4: pp. 477-501.
21. Newman-Tancredi A, Gavaudan S, Conte C, Chaput C, Touzard M, Verriele L, Audinot V, Millan MJ. Agonist and antagonist actions of antipsychotic agents at 5-HT1A receptors: a [35S]GTPgS binding study. Eur J Pharmacol 1998; 355: 245- 56.
22. Hibert MF, McDermott I, Middlemiss DN, Mir AK, Fozard JR. Eur J Med Chem 1989; 24: 31.
23. Kuipers W, Van Wijngaarden I, Kruse CG, Ter Horst-Van AM, Tulp MThM, IJzerman AP. N4-unsubstituted N1-Arylpiperazines as High-Affinity 5-HT1A Receptor Ligands. J Med Chem 1995; 38: 1942-1954.
24. Gaillard P, Carrupt P-A, Testa B, Schambel P. Binding of arylpiperazines, (aryloxy)propanolamines, and tetrahydropyridylindoles to the 5-HT1A receptor: contribution of the molecular lipophilicity potential to three-dimensional quantitative structure-affinity relationship models. J Med Chem 1996; 39: 126-134.
25. López-Rodríguez ML, Ayala D, Benhamú B, Morcillo MJ, Viso A. Arylpiperazine derivatives acting at 5-HT1A receptors. Curr Med l Chem 2002; 9: 443-469.
26. Tammy L, Amanda LB, Andrew M, Caroline Q, Smita P, Kerry Ch, Angus MM. A new class of selective, non-basic 5-HT2A receptor antagonists. Bioorg Med Chem Lett 2006; 16: 3201-3204.
27. ChemDrawn Ultra 9.0. CambridgeSoft. 2004.
28. Frank J, version 2.0, 1993, MOPAC 2.0 Seiler Research Laboratory, US Air Force Academy, Colorado Springs.
29. Tetko IV. Computing chemistry on the Web. Drug Discov. Today 2005; 10: 1497-500.
30. Tetko IV, Gasteiger J, Todeschini R, Mauri A, Livingstone D, Ertl P, Palyulin VA, Radchenko EV, Zefirov NS, Makarenko AS, Tanchuk VY, Prokopenko VV. Virtual computational chemistry laboratory - design and description. J Comput Aid Mol Des 2005; 19: 453-63.
31. Tetko IV, Kovalishyn VV and Livingstone DJ. Volume learning algorithm artificial neural networks for 3D QSAR studies. J Med. Chem 2001; 44: 2411-20.
32. Tetko IV, Luik AI, Poda GI. Applications of neural networks in structure-activity relationships of a small number of molecules. J Med Chem 1993; 36: 811-4.
33. Cavill R, Keun HC, Holmes E, Lindon JC, Nicholson JK, Ebbels TM. Genetic algorithms for simultaneous variable and sample selection in metabonomics. Bioinformatics 2009; 25: 112-118.
34. Yasri A, Hartsough D. Toward an optimal procedure for variable selection and QSAR model building. J Chem Inf Comput Sci 2001; 41: 1218-1227.
35. Chidester CG, Lin C, Lahti RA, Haadsma-Svensson SR, Smith MW. Comparison of 5-HT1A and Dopamine D2 Pharmacophores. X-ray Structures and Affinities of Conformationally Constrained Ligands. Journal of Medicinal Chemistry 1993; 36: 10.
36. Gerber PR. Topological Pharmacophore Description of Chemical Structures using MAB-Force-Field-Derived Data and Corresponding Similarity Measures. In: Carbó-Dorca R, Giromés X & Merzey P eds. Fundamental of Molecular Similarity; Kluwer Academic/Plenum Publishers, New York, 2001, 67-82.
37. STATISTICA 6.0 Statsoft_Inc. 2001.
38. Young DC. Computational Chemistry: A Practical Guide for Applying Techniques to Real-World Problems. John Wiley & Sons, Inc, 2001.
The piperazines are a family of chemical compounds with different pharmacological properties including those arising from the result of interaction with serotonin receptors. The authors, by computational methods, establish a relationship between the structure of the interaction with different piperazines with two types of 5-HT receptor antagonists.
The influence of the substituents of benzene ring as the molecular weight of arilpiperizinas was discussed and a model for undestand the substructures importance in the selectivity of subtype 5-HT was established. Further in vivo studies are needed to confirm the data that the autors observed using computational model.
Comment of the reviewer Prof. Pilar Muñiz Rodríguez PhD. Titular del Área de Bioquímica y Biología Molecular de la Facultad de Ciencias de la Universidad de Burgos. España.
