Indice del volumen Volume index
Comité Editorial Editorial Board
Comité Científico Scientific Committee
MITOCHONDRIAL DYSFUNCTION AND APOPTOSIS IN TROPHOBLAST CELLS DURING PREECLAMPSIA: AN ULTRASTRUCTURAL STUDY
Olivar C. Castejón S.
Laboratorio de Microscopía Electrónica. Facultad de Ciencias de la Salud. Universidad de Carabobo.
Director del Centro de Investigación y Análisis Docente Asistencial del Núcleo Aragua (CIADANA).
Maracay. Venezuela
olivar.ciadanauc @ gmail.com
Rev Electron Biomed / Electron J Biomed 2011;2:30-38.
Comment of the reviewer Alberto Enrique D'Ottavio PhD. rofessor and Researcher, Faculty of Medical Sciences, Rosario National University, Rosario (Argentina).
Comment of the reviewer Larisa Ivón Carrera PhD. Professor and Researcher, Faculty of Medical Sciences, Litoral National University, Santa Fe (Argentina)
SUMMARY
Objective: The aim of this study was to examine the ultrastructure of the mitochondria in trophoblast cells of pregnant women with preeclampsia and to relate it with apoptotic events.
Material and methods: Placentas at term were obtained immediately after cesarean delivery. Three biopsies per placenta were taken from the maternal basal surface in the delivery room. Specimens from peripheral ramifications of the villous tree (especially immature intermediate and terminal villi) were processed through conventional procedures of transmission electron microscopy and opportunely compared with controls obtained from normal placentas.
Results: Ultrastructural degenerative changes in mitochondrial inner membrane and matrix of trophoblast cells (lysis of cristae and matrix vacuolization) were visualized. Swollen mitochondria in diverse degenerative grades were found. Two types of degenerated mitochondria in accordance with the mitochondrial matrix were detected in apoptotic trophoblast cells.
Conclusion: Mitochondrial ultrastructural changes appear to be the origin of trophoblast cell death by apoptosis. Subsequently, trophoblast debris would fall from placental villi into the intervillous space and could become not only corpuscles damaging the endothelium of the fetal-placental maternal unit but one of the stimuli for endothelial dysfunction and the maintenance of the pathogenesis of preeclampsia.
KEY WORDS: Hypoxia. Mitochondrial dysfunction. Trophoblast cell apoptosis. Pre eclampsia
RESUMEN:
Objetivo: Examinar la ultraestructura de la mitocondria en células trofoblásticas de embarazadas con pre-eclampisa y relacionarla con sucesos apoptóticos
Material y métodos: Fueron obtenidas placentas a término inmediatamente después de partos por cesárea. En la sala de partos, fueron tomadas tres biopsias de la superficie basal maternal en cada placenta. Los especímenes, correspondientes a ramificaciones periféricas del árbol velloso (en especial, vellosidades inmaduras intermedias y terminales), fueron procesados mediante técnica convencional para microscopía electrónica de trasmisión y comparados oportunamente con controles obtenidos de placentas normales a término.
Resultados: Se visualizaron cambios ultraestructurales degenerativos en la membrana interna mitocondrial (lisis de las crestas) así como en la matriz mitocondrial (vacuolización matricial). Fueron halladas tanto hinchazón mitocondrial de diversos grados cuanto dos tipos de degeneración mitocondrial, según el estado matricial, en las células trofoblásticas apoptóticas.
Conclusión: Los cambios ultraestructurales mitocondriales parecieran originar muerte celular trofoblástica por apoptosis. Con posterioridad, los fragmentos trofoblásticos caerían desde las vellosidades placentarias hacia espacio intervelloso y devendrían no sólo corpúsculos capaces de dañar el endotelio de la unidad feto-placentaria sino uno de los estímulos para la disfunción endotelial y el mantenimiento de la patogénesis de la pre-eclampsia.
PALABRAS CLAVE: Hipoxia. Disfunción mitocondrial. Apoptosis células trofoblásticas. Pre-eclampsia
INTRODUCTION
Preeclampsia has been correlated with mitochondrial dysfunction in obstetric and gynecological studies and characteristic features of this disorder may be explained by this inborn error in metabolism in which there is a failure in the aerobic energy production1-2.
The genesis of preeclampsia is clearly related to deficient trophoblast invasion and failure of uterine artery remodeling3-4. Defective spiral artery remodeling in preeclampsia likely results in reduced uteroplacental perfusion and foci of placental hypoxia or ischemia5. Bad placental perfusion could cause ultrastructural changes of preeclamptic placental tissue since chorionic hypoxia is a predisposing factor for preeclampsia6.
The ultrastructure of the trophoblast in pregnant women with preeclampsia, recently examined with special reference to the effects of hypoxia, revealed degenerative ultrastructural changes. Swollen mitochondria in diverse degenerative grade were seen with particles electron dense in their matrix7-8.
The mitochondrial damage may be a possible initial cause of trophoblastic apoptosis since hypoxia is apt to produce placental degenerative changes at mitochondrial level. Consequently, a mitochondrial injure could lead not only to the trophoblast cell death but to the detachment of its fragments from placental villous surface after the apoptotic changes associated to hypoxic injury.
Taking into account the aforesaid considerations, this paper reports mitochondrial ultrastructural alterations in apoptotic trophoblast cells of placentas obtained from preeclamptic patients and relates it with apoptotic events.
MATERIAL AND METHODS:
Ten pregnant patients undergoing hypoxia during preeclampsia were strictly studied following basic ethical principles of the Declaration of Helsinki as well as the rules of the Ethical Committee of our institution.
Ten placentas at term were obtained immediately after cesarean delivery. Three biopsies per placenta were taken from the maternal basal surface in the delivery room. Two to five mm specimens from peripheral ramifications of the villous tree (especially immature intermediate and terminal villi) were instantly fixed in 4% glutaraldehyde - 0.1 M phosphate buffer, ph 7.4, at 4°C. After its division in one mm each, they were immersed in a fresh similar solution from 2 to 72 hours followed by a secondary fixation in 1% osmium tetroxide - 0.1 M phosphate buffer for 1 hour. Subsequently, samples were processed through the conventional procedures for transmission electron microscopy7. Electronic micrographs were selected and compared with those of normal placentas at term (controls).
Criteria related with tissue hypoxia were complementary taken into account6.
RESULTS
Presence of villous trophoblast with degenerative changes surrounding the peripheral area of placental villi was put into evidence. Increased syncytial knots, syncytial sprouts, infarction of villi, apoptotic trophoblast cells, intervillous thrombi and intervillous deposit of fibrinoids were also observed when semi-thin sections of biopsies were analyzed.
Regions of cytotrophoblast revealed dilated cisterns of rough endoplasmic reticulum. This organelle presented degranulation of ribosomes in its membranes. This was not seen in controls. A light hyaloplasmic and nucleoplasmic matrix was observed. In some cases scarce heterochromatin was noted associated to inner nuclear membrane. Interrupted cytoplasmic microfilaments were dispersed in the hyaloplasmic matrix of cytotrophoblast cell. Swollen electron-dense mitochondria were visualized with interruption of membranes, cristolysis and obscure mitochondrial matrix. In control specimens, the integrity of the mitochondrial ultrastructure was preserved.
Regions of syncytiotrophoblast in process of degenerative change were found. These regions were more abundant in preeclamptic specimens. Some cytoplasmic prolongations of cytotrophoblast cells were more obscure and located between the cytotrophoblast cell and basal membrane. This appeared thickened and penetrated by collagen fibers. Different thickness was noted in their trajectory (Fig 1).

Fig.1.- Region of cytotrophoblast cells. Mitochondria show notorious electron density changes in mitochondrial matrix probably due to
mitochondrial matrix protein aggregation responsible for its osmiophilic property.
Collagen fibers are seen incrusted in trophoblast basement membrane in the lower right-hand side of the micrograph. Bar: 1 µm
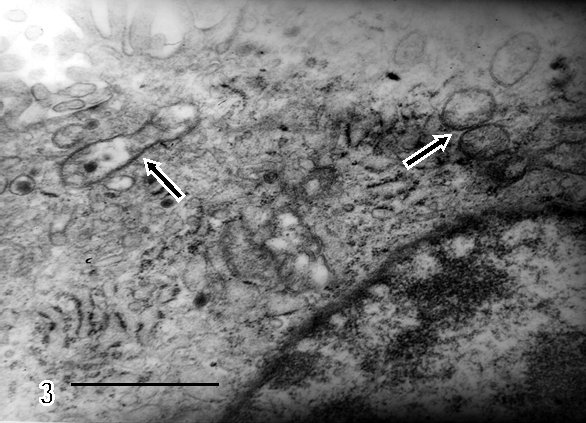
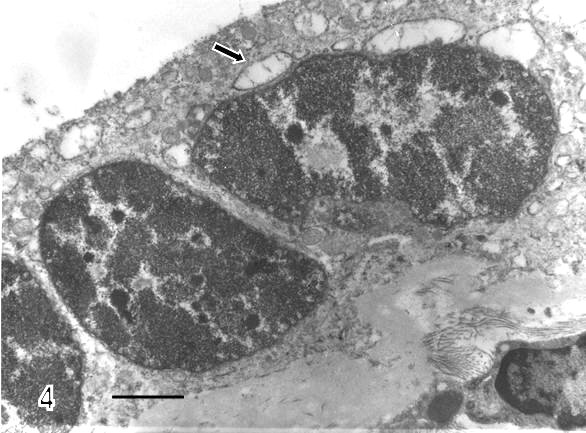
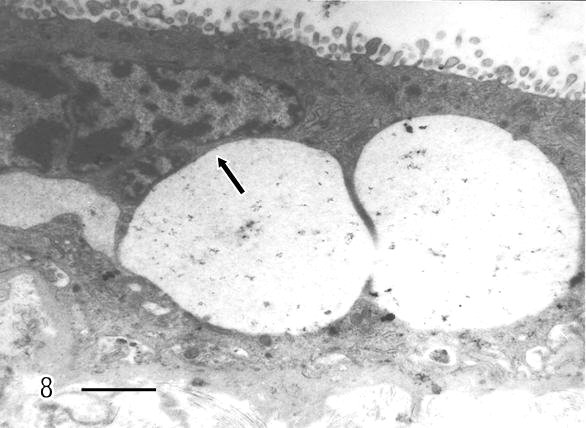
There are zones of cytotrophoblast cells that contain two types of mitochondria, ones are of clear mitochondrial matrix and others of electron dense mitochondrial matrix observed in degenerating trophoblast cells (Fig 2). The syncytiotrophoblast also showed swollen mitochondria with strongly osmiophilic intramitochondrial granules. These mitochondria had interrupted crystae and exhibited vacuolar zone in their light matrix (Fig 3) whilst in controls images of mitochondria preserved the normal ultrastructure. The syncytiotrophoblast presented an interrupted plasma membrane, a vacuolar cytoplasm and apoptotic nucleus (Fig 4). These features were not observed in most of control specimens.
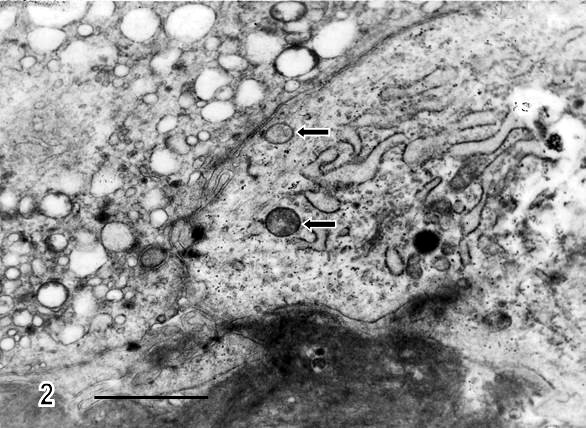
Fig.2.- Thickened and disorganized basal membrane is observed in regions of trophoblast cells.
The cytotrophoblast contains two types of mitochondria (arrows) which differ in electron density from the mitochondrial matrix.
A region of vacuolated syncytiotrophoblast is visualized in the upper left-hand side of the micrograph. Bar: 1 µm
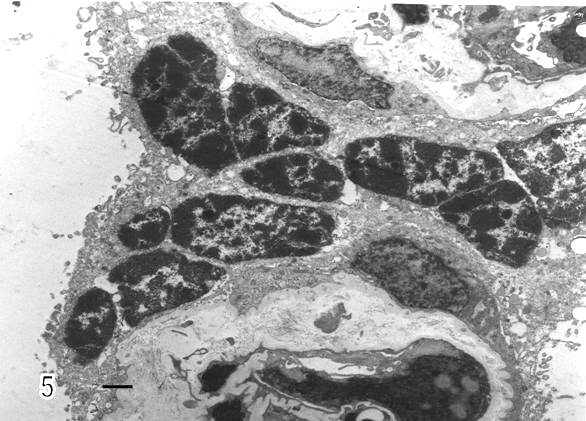
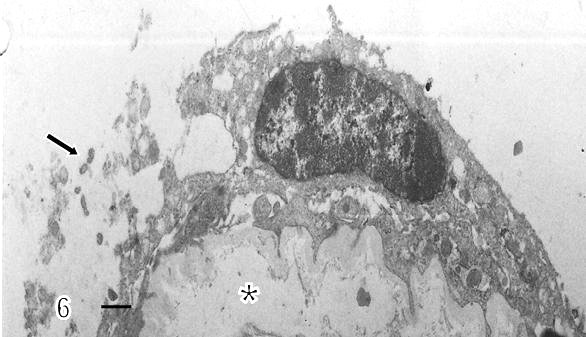
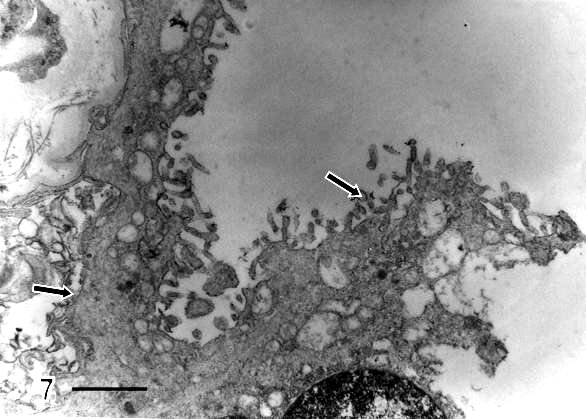
Syncytial knots were observed with plasma membrane showing microvilli in blebs, filamentous or battledore form (Fig 5). These were increased in preeclamptic specimens when compared with controls. Zones detached of trophoblast cell were seen in placental villi which presented subtrophoblastic edema (Fig 6). Large areas of degenerating trophoblast appeared to be delivered to the intervillous space (Fig7). Fragments of syncytiotrophoblast were expulsed to the maternal circulation by a process of subtrophoblastic edematous change (Fig 8).
DISCUSSION AND CONCLUSION
Several electron microscopic studies have suggested that lesions of placental tissue in preeclampsia could be discussed on the basis of changes in endothelial cells or reduced perfusion by maternal blood9-13.
No characteristic findings were found associated to preeclampsia. Mitochondrial changes have been seen in vessels, myometrial smooth muscle, myometrial interstitial cells, circulating leukocytes, epidermal and dermal cells and in hepatocytes of preeclamptic pregnant women14. The mitochondria of these preeclamptic tissues showed a central disruption. Similar structural studies of mitochondria in cerebral edema and brain ischemia or anoxia have also been reported15. The injured mitochondrial patterns are related with nerve cell death and postulated as markers of lethal nerve cell injury. Mitochondrial injury relates with hypoxic-ischemic processes and precedes trophoblastic apoptosis6,9.
Reduced utero - placental flood flow has been recognized in cases of severe preeclampsia with hypertension6. In these cases, histological changes as decreased number of syncytial microvilli, proliferation of cytotrophoblastic cells, focal syncytial necrosis, thickening of trophoblastic basement membrane and narrowing of fetal capillaries have been demonstrated8.
In this study, mitochondrial degenerative changes occur in trophoblast cells undergoing apoptosis. Chronic hypoxia or alternate periods of hypoxia/re-oxygenation within intervillous space is expected to trigger tissue oxidative stress and increase placental apoptosis16. Mitochondria contain the enzymatic systems which generates ATP through the Krebs cycle and oxidative phosphorylation. The formation of ATP requires oxygen which diminishes in preeclampsia. In this condition, it is suggested that trophoblast cells undergo apoptotic events and fragments or debris are delivered into the intervillous space 7.
Previous reports pointed out that an increased release of syncytiotrophoblasts microparticles (STBM) of 0.2 to 2um in size formed by plasma membrane blebbing during apoptosis are triggered in excess into the maternal circulation17,18. The present findings are congruent with those reported by Bachmaier et al19. They also found swollen and degenerating mitochondria in hypoxia condition at 34 mmHg of oxygen pressure according to their model of hypoxic dual in vitro placental perfusion.
Thus, mitochondrial death induces trophoblastic apoptosis in degenerating placental villi. The role of the mitochondria in apoptotic cell death is well known. Hypoxia acting as an external stimulus changes the mitochondrial function. Mitochondria discharge cytochrome c, this protein actives the system of caspases and the trophoblast die by apoptosis. The increased apoptosis in preeclampsia leads to greater number of syncytiotrophoblastic fragments being shed into the maternal blood20. These detached fragments, debris of trophoblast, microvilli from syncytium or syncytial microparticles could be considered as causing endothelial damage, among other factors. Syncytiotrophoblastic detritus detached from the trophoblastic surface and resulting from a continuous process of regeneration, reparation or apoptosis are increased during preeclampsia. Being a consequence of a poor blood supply subsequent to a spiral artery failure occurring in placental implantation, it constitutes the stimulus for a permanent inflammatory response characteristic in this common and dangerous pregnancy complication21.
During preeclampsia, the machinery maintaining the intracellular respiration and the biosynthesis of ATP in the trophoblast is damaged. The trophoblast is then transformed in a tissue with tendency to be fragmented in smaller particles passing initially to the intervillous space and subsequently to the endothelium of the fetal- placental maternal unit. These fragments of tissue contacting maternal endothelium could stimulate the process of disseminated intravascular coagulation, typical of preeclampsia. Lapaire et al. (2007) reported that Schmorl in 1893 was one of the first researchers in recognizing the placental origin of preeclampsia22. These results obtained from transmission electron microscopy support it 118 years after.
In conclusion, these findings could be pointing out that in preeclampsia the trophoblast undergo apoptosis caused by mitochondrial damage, transforming it in a tissue with tendency to be fragmented. Such detritus detached from their surface could become the stimulus for endothelial dysfunction during the maintenance of the analyzed pregnancy complication.
REFERENCES:
-
1. Widschwendter M, Schrocksnadel H, Mörti M G. Preeclampsia: A disorder of placental mitochondria. Mol Med Today 1998; 4: 286-291
2. Torbergsen T, Olan P, Mathlesen E, Bernd O. Preeclampsia: A mitochondrial disease. Acta Obstet Gynecol Scand 1989; 68: 145-148
3. Kiswara S, Ara S, Abu Rayhan K, Begum M. Morphological changes of the placenta in preeclampsia. Bangladesh J Anat 2009; 7:49-54
4. Hawfield A, Freedman BI. Preeclampsia: The pivotal role of the placenta in its pathophysiology and markers for early detection. Ther Adv Cardiovasc Dis 2009; 3:65-73
5. George EM, Granger JP. Recent insights into the pathophysiology of preeclampsia. Expert Rev Obstet Gynecol 2010; 5:557-566
6. Robinson NJ, Wareing M, Hudson NK, Blankley RT, Baker PN, Aplin JD et al Oxygen and the liberation of placental factors responsible for vascular compromise. Lab Invest 2008; 88:293-305
7. Castejón S O C, López A J, Castejón M O C. Cambios ultraestructurales del trofoblasto en los casos de hipoxia durante la preeclampsia. Rev Obstet Ginecol Venez 2008; 68: 168-174
8. Salgado SS, Salgado MKR. Structural changes in preeclamptic and eclamptic placentas-an ultrastructural study. J Coll Physicians Surg Pak 2011; 21:482-486
9. Illsinger S, Janzen N, Sander S, Schmidt KH, Bednarcz KJ, Mallunat L et al. Preeclampsia and Hellp syndrome: impaired mitochondrial function in umbilical endothelial cells. Reprod Sci 2010; 17:219-226
10. Hupertz B, Kingdom J, Caniggia I, Desoye G, Black S, Kerr H, et al. Hypoxia favorous necrotic versus apoptotic shedding of placental syncytiotrophoblast into the maternal circulation. Placenta 2003; 24: 181-190
11. Sulbarán TA, Castellano A, Chacin L, Vergel C, Portillo MA, Urbina E et al. Electron microscopy of umbilical cord endothelial cells in preeclampsia. J Submicroscopic Cytol Pathol 2002; 34:389-395
12. Hirano H, Imai Y, Ito H. Spiral artery of placenta: development and pathology-immunohistochemical, microscopical and electron microscopy study. Kobe J Med Sci 2002; 48:13-23
13. De Luca Brunori J, Battini L, Brunori E, Lenzi P, Paparelli A, Simonelli M et al. Placental barrier breakage in preeclampsia: Ultrastructural evidence. Eur J Obstet Gynecol Reprod Biol 2005:118:182-189
14. Dokras A, Hoffmann DS, Eastvold JS, Kienzie MF, Gruman LM, Kirby PA et al. Severe feto-placental abnormalities precede the onset of hypertension and proteinuria in a mouse model of preeclampsia. Biol Reprod 2006; 75: 899-907
15. Castejon OJ. The thesis of mitochondria as marker of lethal injury in the traumatic human brain oedema. An electron microscopic study using cortical biopsies. Acta Microscopica 2008; 17: 16-27
16. Grill S, Rusterholz C, Zanetti-Dallenbach R, Tercanli S, Holzgreve W, Hahn S et al. Potential markers of preeclampsia-a review. Reprod Biol Endocrinol 2009;7:70-75
17. Guller S. Role of the syncytium in placenta-mediated complications of preeclampsia. Thromb Res 2009; 124: 389-392
18. Redman CW, Sargent IL. Circulating microparticles in normal pregnancy and pre-eclampsia. Placenta 2008;29A:S73-S77
19. Bachmaier N, Linnemann K, May K, Warzok R, Kuno S, Niemeyer M, et al. Ultrastructure of of human placental tissue after 6 h of normoxic and hypoxic dual in vitro placental perfusion. Placenta 2007; 28: 861-867
20. Levy R. The role of apoptosis in preeclampsia. IMAJ 2005; 7: 178-181
21. Goswami D, Tannetta DS, Magee LA, Fuchisawa A, Redman CWG, Sargent IT, et al. Excess syncytiotrophoblast microparticle shedding is a feature of early-onset preeclampsia but not normotensive growth restriction. Placenta 2006; 27: 56-61
22. Lapaire O, Holzgreve W, Oosterwijk J C, Brinkhaus R, Bianchi D W. Georg Schmorl on trophoblasts in the maternal circulation. Placenta 2007; 28: 1-5
ACKNOWLEDGMENTS:
I am deeply grateful to the delivery room staff at the Maracay Central Hospital for their help in obtaining placentae; Mr. Raúl Colina and Milagros Díaz by technical assistance in the laboratory of electron microscopy of the Odontological Research Institute-Caracas and to Dr Gilberto Sánchez of the Venezuelan Institute of Scientific Research- Los Teques who facilitated the infrastructure of transmission electron microscopy; and to the Administrative Coordination of the Health Sciences Faculty of the Carabobo University of Nucleus Aragua Venezuela by financial support for CIADANA.
CORRESPONDENCE:
Prof. Olivar C Castejón. Prof. Titular en Biología Celular. Director del CIADANA
Laboratorio de Microscopía Electrónica. Facultad de Ciencias de la Salud.
Universidad de Carabobo - Núcleo Aragua.
Centro de Investigación y Análisis Docente Asistencial del Núcleo Aragua (CIADANA).
Maracay, Edo. Aragua. Venezuela. Apdo. 4944.
Mail: olivar.ciadanauc @ gmail.com