Indice del volumen Volume index
Comité Editorial Editorial Board
Comité Científico Scientific Committee
EFFECT OF BROMINE ATOMS NUMBER ON THE
CYTOTOXICITY OF TWO 2-FURYLETHYLENE DERIVATIVE SUBSTANCES
IN NORMAL AND TUMORAL CELL LINES.
1Oscar Hernández PhD, 1Yudiska Martinez MSc, 2Héctor Pimentel MSc, 1Llanetsy Llanes, 3Giselle Pérez PhD, 1Nayadis Vazquez, 1Sandra Fernández, 1Quesada Lidice, 1Aime Debesa
1Centro de Inmunología y Productos Biológicos (CENIPBI),
Universidad Médica. Camagüey.
2Centro Provincial de Genética Médica. Camagüey.
3Centro de Bioactivos Químicos (CBQ).
Universidad Central de Las Villas. Villa Clara.
Cuba
oscar.hernandez1964 @ yahoo.es
Rev Electron Biomed / Electron J Biomed 2012;1:26-36
Comment of the reviewer Prof. Pilar Muñiz Rodríguez PhD. Titular del Área de Bioquímica y Biología Molecular de la Facultad de Ciencias de la Universidad de Burgos. España.
Comment of the reviewer Prof. Francisco Abad Santos Clinical Pharmacology. Hospital la Princesa. Departamento de Farmacología y Terapéutica de la Facultad de Medicina. Universidad Autónoma de Madrid. España
SUMMARY:
The study was performed to investigate the effect of bromine atoms number present in two tested substances derivatives of 2-furylethylene on cell proliferation. The substances carrying one or two Br atoms were coded as MA and G1 respectively. The neutral red uptake (NRU) assay and mitotic index (MI) were used for this purpose.
The presence of two bromine atoms on the molecule of G1 inhibited markedly the cytotoxicity of this composite. For CHO cell line, the IC50 values were 256.6 µM for G1 and 134.5 µM for MA; whereas in SK MEL-3 (human melanoma) cell line, the IC50 were 413.4 µM and 264.1 µM for G1 and MA respectively. The IC50 values obtained in both cell lines were higher than 100 µM and showed no specificity for tumoral cells.
The MI obtained with the G1 composite showed no significant differences with phytohaemoglutinine used as positive control. The anti-proliferative effect and MI were related with the number of bromine atoms on the molecules assayed. Another experiment was conducted with the MA product to obtain information about the acute oral toxicity class methods. The tested compound was classified in the 3th toxicity class with a fixed LD (50) cut-off value of 200 mg/kg of body weight.
KEYWORDS: Bromine, Cytotoxicity, Acute toxicitybromine, Mitotic index. Furylethylene, CHO.
RESUMEN: EFECTO DEL NÚMERO DE ÁTOMOS DE BROMO EN DERIVADOS DEL 2-FURILETILENO SOBRE LA CITOTOXICIDAD DE LÍNEAS CELULARES NORMALES Y TUMORALES.
El objetivo del estudio fue investigar el efecto que tiene el número de átomos de bromo presente en dos sustancias derivadas del 2-furiletileno sobre la proliferación celular. Las sustancias portando uno o dos átomos de bromo en su molécula se codificaron como MA y G1 respectivamente y su efecto sobre las líneas celulares se evaluó mediante las técnicas de captación de rojo neutro (CRN) y del indice mitótico (IM). La presencia de dos átomos de bromo en la molécula del G1 inhibió marcadamente la citotoxicidad de este compuesto, para la línea celular CHO (ovario de hámster chino) los valores de IC50 fueron 256.6 µM para el G1 y 134.5 µM para el MA; mientras que en la línea SK MEL-3 (melanoma human), los valores de IC50 fueron 413.4 µM y 264.1 µM para el G1 y MA respectivamente. Los valores de IC50 obtenidos por estos compuestos en ambas líneas celulares fueron superiores a 100 µM y no mostraron especificidad por la línea tumoral.
El IM obtenido con el compuesto G1 no mostró diferencias significativas con la fitohaemoglutinina, empleada como control positivo del ensayo. El efecto anti-proliferativo y el IM estuvieron relacionado con el número de átomos de bromo en las moléculas ensayadas. Por otra parte un estudio llevado a cabo con el compuesto MA brindó información sobre la toxicidad aguda del mismo utilizando el método de las clases, clasificándose este producto en la clase 3 con un valor de corte LD (50) de 200 mg/kg de peso corporal.
PALABRAS CLAVE: Bromo. Citotoxicidad. Toxicidad aguda. Índice mitótico. Furiletileno. CHO.
INTRODUCTION
Since the 1990's, 2-furylethylene derivatives have been regarded as an interesting range of biological active compounds. They are one of the useful classes of synthetic agents with a wide range of biological activities including, antibacterial, antifungus and antiprotozoal properties1-4. The 2-furylethylenes, also known as vinylfurans or ethenylfurans, are derivatives of the ethene where a furan ring is attached to one of its carbon atoms (the ? carbon). The presence of a nitro group at position 5 of the furan ring in most of the 2 furylethylenes reported in literature until the 1980's, limited the use of these compounds because the aromatic nitro compounds in general, and nitrofurans in particular, have mutagenic and carcinogenic properties5-6. Subsequent studies opened a novel opportunity for these compounds, because it demonstrated that the presence of a nitro group at position 5 of the furan ring is not a necessary condition for the development of the antimicrobial activity of such chemicals7-8.
The (1-(5-bromofur-2-yl) nitroethene) and (1 (5 bromofur 2 yl) 2 bromo 2 nitroethene) coded for this paper as MA and G1 respectively are a synthetic 2-furylethylene derivatives. They have one or two bromine atoms added into the molecule and were used in this study in order to compare their effect on the biological activity changes. The addition of bromine atoms in different combinations could cause diverse responses due to the atomic mass of this element (79,904). The position and number of the bromine atoms on the structure of several natural9-11 and synthesized12-13 compounds has attracted the attention of many biomedical research in order to use these compounds in future human health treatments. Some of these works show that bromination and the position of this atom over those substances increased the cytotoxicity of the compounds tested14-15. Other results have shown that chemical modifications like a substitution of bromine or iodine has a significant effect on the binding properties of the product to DNA16-17.
There has been recently an increasing interest in controlling macromolecular conformations and interactions through the adding of halogen bonding to sensitized compounds. Halogen bonds are favorable electrostatic interactions between polarized, electropositive chlorine, bromine or iodine atoms. An halogen bond is a mainly an electrostatic interaction between a classic hydrogen bond acceptor, such as the electronegative O, N or S18. This sort of bond seems to play a role in binding and recognition similar to that in the thyroid-related hormones, a diversity of short X···O interactions (X = halogens) that are involved in protein/ligand recognition19.
The in vitro systems are ideally suited for investigations of the molecular, cellular and physiological mechanisms of chemically induced toxicity; the main justification for developing in vitro toxicity tests is that they will make toxicology a more scientifically based practice. It is even more obvious that the development and incorporation of stepwise testing strategies, combining experimental data from a range of alternative methods (metabolic and kinetic modeling, quantitative structure-activity relationships - QSAR, and in vitro tests), provide the most advanced way to predict toxicity, reducing at the same time the number of laboratory animals used for testing.
Moreover, the costs of assessing potential health effects of around 200,000 substances per year that are newly identified or synthesized require alternatives methods of analysis. But we must be sure that the obtained results with these alternative methods are reliable in order to substitute or reduce the number of tests in animals. In this investigation we used an in vitro system as an alternative method to evaluate the bromine atoms number effect on cellular toxicity of these 2-furylethylene derivatives using normal and tumoral cell lines.
MATERIAL AND METHODS.
The experiments were carried out at the Molecular and Cell Biology Department and at the Animal Housing Laboratory of the Centro de Inmunología y Productos Biológicos (CENIPBI) at the Universidad de Ciencias Médicas "Carlos J. Finlay" in Camagüey, Cuba.
1.-Synthetic product used in the trail
The substances tested were coded as MA 1-(5-bromofur-2-yl) nitroethene and G1 (1 (5-bromofur-2-yl)-2-bromo-2-nitroethene) a 2-furylethylene derivatives synthesized and gently offered by the group of chemical synthesis of the Centro de Bioactivos Químicos (CBQ), at the Universidad Central de Las Villas, Santa Clara, Cuba. Its global formulas are: C6H4BrO3N and C6H3Br2O3N for MA and G1 respectively. Both of them consisted of a yellow crystalline powder and its purity was certified by analytical testing to be higher than 99.0% in both cases. The molecular formulas of these composites are showed below:
2.-Cell lines and culture conditions
To assess the cytotoxic effect of the synthesized composite we used CHO-K1 cell line (Chinese Hamster Ovary), purchased from ECACC (Nº85050302), and SK-MEL-3 (ATCC NºHTB-69) a human melanoma cell line, kindly given by the Centro de Inmunología Molecular, Havana, Cuba. RPMI 1640 medium (Gibco 31800-014) supplemented with 10% Fetal Bovine Serum (Gibco-BRL, Grand Island, NY, USA) and 2 mM glutamine piruvate without antibiotic was used for this cell line.
The cellular suspensions were obtained from a 25 cm2 flask at 70-80% final confluence. The detached cells by tripsine solution treatment (0.125%, 53 mM EDTA in PBS 1X) during 3 5 minutes at 37°, were counted in a Neubauer camera using the trypan blue dye exclusion test.
The viability was higher than 90% in all assays. The cells density used varied between 20 and 30×103 cells/well in 96 wells plates (COSTAR N°-402037310) seeded at 100 µl final volume. The plates were incubated in 100% humidity and 5% CO2 atmosphere at 37°C, during 24 hours before the treatment application to permit the cellular adhesion to the bottom plate.
3.- Animals
Wistar Rats, SPF from Laboratory Animals Breeding Center, CENPALAB, (Cuba). Age: 35 - 41 days, weight 101-125 g. Microbiological status: free from ecto and endoparasites, mycoplasms and pasteurellae. 30 females were supplied by airplane in rats boxes and acclimatized (22 ± 3 °C; 50 ± 10%) for 1 week in the Animal Housing Laboratory (CENIPBI).
During the experiment, animals were housed in polycarbonate cages Type 4 (3 rats/cage) on sterilized wood shavings as a bedding. Room temperature and humidity were regulated at 22 ± 3 °C and 50 ± 10%). Lighting conditions: Fluorescents lamps, with a sequence of 12 hours of light and 12 hours of dark. Air flow rate 0,5 m/s above cages.
Feeding: A commercial diet (8 mm pellet) manufactured by CENPALAB, Havana, Cuba (ISO 9001 Certified Laboratory) was heated for 20 min at 110 °C, and available ad libitum. Drinking water: acidic sterilized water, ad libitum.
Experiment performed from July 7 to July 22, 2010 using 9 rats/experiment.
The behavioral parameters were measured as indicated by OECD 423 guideline.
4.-Experimental Design
4.1.-Cytotoxic Test
The neutral red uptake (NRU)20 was selected from the different in vitro tests described to evaluate the cytotoxicity. Prior to each experiment the test substances were dissolved in a (DMSO/ETOH) (v/v) mix and immediately diluted into medium. During drug exposure the concentrations of DMSO/ETOH mix, in treated and control wells, were kept below 0.1%. We prepared a 500 mM main solution of the product to then make the following doses: 400, 300, 250, 200, 100 and 50 µM afterwards, the doses and control solutions were filtered using 0.45 and 0.22 µM membranes. The negative control was the RPMI medium with 0.1% of the diluent used (DMSO/ETOH) without the test substances. As a positive control 10 ?g/ml of 5-fluorouracile (5-FU), was used. The tests were carried out in triplicate (3 wells/dose) during 72 hours. To keep the optimal concentrations and cultural conditions 100 µl of the medium were changed daily, containing the adequate doses, including controls. The optical density was recorded in an ELISA reader plate equipment (Lab Systems MULTISKAN Plus) at 540 nm wavelength. A lineal regression method was used to determine the IC50 value21-22, using Prism; GraphPad Software (5.02 version).
4.2.-Determination of Mitotic Index (MI)
The mitotic index was used to determine the cell division rate and to compare the potentiality of MA and G1 to inhibit mitosis. To allow the occurrence of more than one division cell cycle, the product concentrations used in the MI test was below the IC50 value determined in previous cytotoxicity experiments (50 and 150 µM for MA and G1 respectively). We used a CHO cell line for this test and it was performed in 35 mm dishes (MERCK 402/0455/02) using three dishes by treatment. After incubating for 48 hours at 37 ? and 5% CO2, the cells were detached by means of a 0.125% tripsine solution and the Verma and Babu (1995) protocol was used to obtain the metaphases23.
Randomly selected views and non-overlapping cells on the slides were monitored to determine the number of dividing cells (metaphase stage) and the total number of cells using an Olympus BX51 microscope. 1000 cells/dish were examined in each treatment. Mitotic index values are expressed as% in this study by means of the formula showed below.
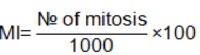
As a positive control we used 180 µg/ml of mitotic inductor phytohemoaglutinine (REMEL-E, HA15/3085 2701), in the concentration suggested by the supplier.
4.3.-Acute Toxic Class Method
The only product used in this test was MA, it was dissolved in 1 ml of vegetable sunflower oil. The Acute Toxic Class Method began with 300 mg/kg body weight, as indicates the OECD 423 guideline in the case of absence of previous data of toxicity. Taking into account the 300 mg/kg b. wt. results we needed to administer a second dose of 50 mg/kg b. wt. The test substance was administered in a single dose by gavage using a suitable intubation canula. The negative control was constituted by a group of 3 animals that only received sunflower oil without the test substance. Once the administration of the product began, we observed the animals during the first four hours with special attention, in the first 30 minutes.
During 14 days after administration, clinical examinations were performed daily, and body weights were measured before dosing and on days 1, 7, and 14, according to the guidelines previously mentioned. The animals were checked for mortality, general state, external appearance, behavior, and clinical symptoms like: itch, dyspnea, depositions, skin and mucous irritation, violent disturbances, and others.
All signs and symptoms were tabulated for each animal including the hour and date when they started and/or disappeared. The date and hour of animal death during experiment were also registered. At the end of this period (14 days), the live animals were euthanized by injecting an overdose (three times the anesthetic dose) of sodium thiopental (60 mg/kg b. wt.) intraperitoneally and necropsied. The organs from animals that died and from those that survived were collected for gross macroscopic examination of organs and tissues (heart, kidney, spleen, lung, liver and stomach), which was performed at the Anatomy Department at the Regional Veterinary Clinic in Camagüey, Cuba.
5.-Statistical analysis
The statistical analysis was performed with SPSS 15.0 for windows. The data were submitted to a parametric one-way analysis of variance test (ANOVA) and the Tukey's multiple comparison post-tests. The data were expressed as the means ± S.D. of three independent determinations.
To determine the statistical significance between treatments at Mitotic Index assay we performed a non parametric test (proportions comparison test). The differences were considered significant at p<0.05.
RESULTS
1.- Citotoxicity
We wanted to compare in vitro cytotoxicity of two out of five members of the family taking into account the quantity of bromine atoms on the molecular structure (Figure 1).
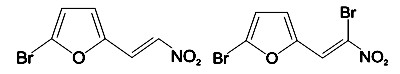
Figure 1. Molecular structure of MA (left) and G1 (right) test substances.
The G1 composite contains two atoms of this element, one on the ring and the other in position 2 of the lateral carbon chain outside the furan ring; on the other hand MA has only one bromine atom at the furan ring.
Figure 2 and 3, show the dose response curves for both products in CHO and SK-MEL-3 cell lines respectively.
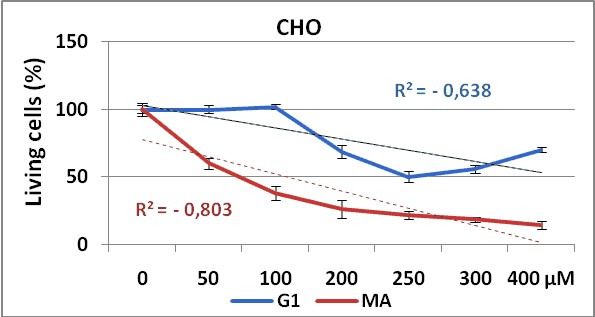
Figure 2. Dose response curve obtained by G1 (blue line)
and MA (red line) test substances in CHO cell line using NRU method.
Experiments were made in triplicate during 72 hours. Each point represents ± SD
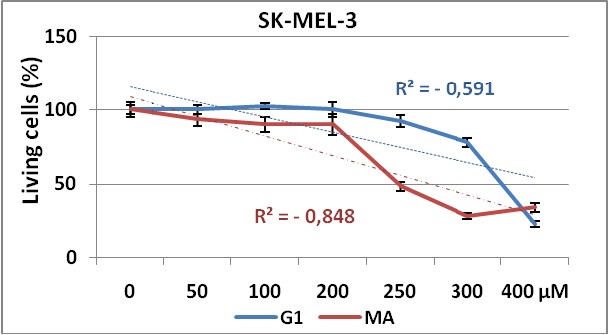
Figure 3. Dose response curve obtained by G1 (blue line)
and MA (red line) test substances in SK-MEL-3 cell line using NRU method.
Experiments were made in triplicate during 72 hours. Each point represents ± SD.
The use of two different cell lines gave us information about the effectiveness of the test substances (G1 and MA), against cell lines from different tissues. Our results demonstrate that both cell lines show dose-dependent response to these substances administrations, with significant differences in proliferative responses between MA and G1 (p=0,021) in CHO cell line. But surprisingly, in SK-MEL-3 cell line (figure 3) no significant differences were shown (p=0,335) on cell proliferation inhibition between these compounds, maybe due to a higher resistance of this tumoral cells to the treatment with this composites as observed in others experiments24.
The growth inhibitory activity as IC50 of compounds G1 and MA evaluated in both cells lines are represented in Table 1.
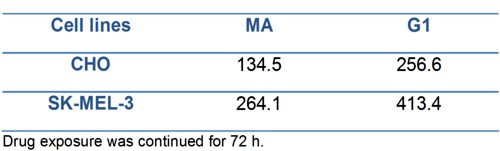
Table 1.- IC50 (µM) values for compounds MA and G1 determined by lineal regression.
We can observe in both cell lines the higher anti-proliferative effect of MA product showing the lower IC50 value, when comparing with G1.
On the other hand, the higher IC50values obtained for SK-MEL-3 (a human melanoma cell line) compared with the IC50obtained in CHO (non tumor cell line) evidenced first the non tumor specific action of these products over the tumoral cell line here used, and secondly, the low efficacy of these synthesized compounds taking into account the high (> 100 µM) concentrations needed to be 50% cytotoxic in both cell lines.
2.- Mitotic Index
Mitotic index is usually represented as the percentage of cells undergoing division of the total number of cells. For those reason two members of the 2 furylethylene family with one and two bromine atoms (see figure 1) were employed to compare the bromine atom number effect on the cell cycle division.
Figure 4 represents the media values of mitotic index obtained from experiments made in triplicate with their standard deviations.
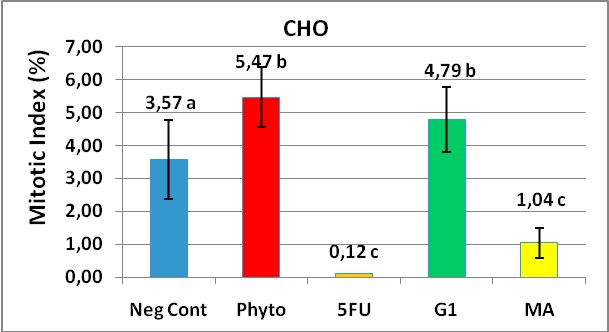
Figure 4. Mitotic Index (MI) values to assess a cellular division
inhibition in CHO cells treated for 48 hours with MA and G1. 1000 cells were counted
in each treatment and the MI was calculated by a formula showed in materials and methods.
The stimulating effect (5,47%) of phytohaemoglutinine, a commercial mitotic inductor at 180 ?g/ml was evidenced when we compared it with cells growing in presence of the diluent vehicle without the test substances as a negative control (3,57%). Interesting results were to compare the MI between MA and G1 products. Surprisingly on the G1 product which has a two bromine atoms, showed significantly to stimulate the CHO cellular division compared with the negative control (p = 0.001). On the other hand the MA substance with only one bromine atom linked to the furan ring, markedly decreased the mitotic index, a useful marker of cell cycle division inhibitor and showed a significant deference with G1 (1,04 vs 4,79 respectively).
3.-Acute Oral Toxicity Test
Different 2-furylethylene derivatives products are in studies by their antifungus and antimicrobial properties among others but, the MA product once of the most recent derivative obtained does not present any toxicological data. The results obtained recently with this product at in vitro cytotoxicity test incentive us to carry out the preclinical studies to obtain toxicological information about it, performing the acute oral toxicity class methods.
3.1.-Clinical manifestations as toxicity criteria
At doses of 300 mg/kg b.wt., all animals presented a series of toxic signs. It goes from loss of balance, loss of mobility, respiratory upsets (dyspnea), lethargy, pilo erection and cyanosis, up to the coma and prostration until to reach death. All these rats died between 2 and 6 hours following administration of MA product. Gross necropsy revealed some evidence of acute gastritis and gastrointestinal tract distention. Congestion and some little hemorrhagic area in the lungs were found but not bleeding and intestine distention was consistently found in one individual. All controls survived the 14 days trial and did not exhibit gross evidence of hemorrhage.
We proceeded to perform another experiment with a lower doses recommended by guideline. Few minutes after doses application of 50 mg/kg b.wt., the animals presented some light signs as respiratory discomfort and itch which disappeared two hours after. In this case the product did not cause any death. The surviving rats were sacrificed on day 14 of the experiment. At necropsy, the rats were in good body condition, and neither macroscopic damages were observed on the organs selected for study nor evidence of starvation or dehydration.
To confirm the results obtained with 50 mg/kg b.wt. doses, and as recommended by guidelines (OECD423) we performed another experiment at the same doses.
The statistical results for p<0.05, show no significant differences between the groups in any of the studied organs. Together with the anatomic pathologic information registered, it corroborates the non toxicity of this product to these doses of 50 mg/kg b.wt.
3.2.-Body weight as toxicity criteria
Body weight data are depicted in figure 5. The ANOVA and Tukey's post test performed for n=3 and p<0,05 showed no significant differences (p=0,341) in body weight among groups. However, we found significant differences (p = 0,002) in the body weight gained between the experiment start day (day zero) and the day 14, which makes evident the non toxicity of the MA product at this dose.
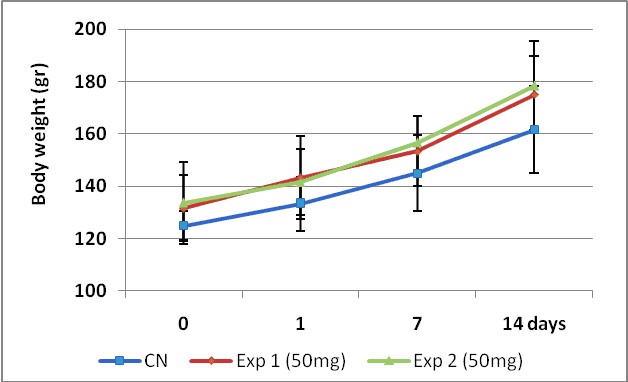
Figure 5. Growing curves comparison between control and two experiments carry out with 50 mg/kg b.wt.
of MA test product by 14 days. The experiment was performed following the OECD 423 guidelines.
To determine significance differences between groups the ANOVA and Tukey's post test was performed for n=3 and p<0,05.
Body weight media value ± SD are show.
According to the acute toxic class method, the tested compound was classified into the third toxicity class (>50-300 mg/kg b. wt.) with a fixed LD50 cut-off value of 200 mg/kg b.wt.
This in vivo toxicity results well corroborate the in vitro outcomes using cell lines, because in previous studies (no published results) performed in rat, the LD50 of G1 product showed a significant higher value (1856.3 mg/ kg b.wt.) compared with MA (200 mg/kg b.wt.) using acute toxic class method.
Although it is certain that we employed two different methods to evaluate the in vivo toxicity by using rats, is obvious that the monobrominate product (MA) is quite more toxic than the product that carries two atoms of bromine in their structure (G1).
DISCUSSION
It is known that several aromatic nitro compounds are mutagenic inductors as the result of electrophilic attack of the purinic bases in DNA by the activated nitrogen, which causes chemical modifications over DNA molecule25. In the case of the 2-furylethylene derivatives tested, a nitro group placed outside of the furan ring generates significant changes in the physical chemical, chemical and biological properties, different from the 5-nitrofurylethylen composites1.
The addition of specific chemical groups to the 2-furylethylene derivatives in preceding studies had the purpose of investigate the specific variations of biological properties (bactericide, antifungal or antitumoral). The bactericide and antifungal activity of this family have been proved1,3. However, by using cell lines the cytotoxic effects evidencing the antitumoral potential of these products have not been demonstrated yet.
Two main properties characterize the wide number of well established antitumor commercial agents or even under researches at present. First, its need to be highly specific by tumor cell lines and second its most shows a very low IC50values in the range of nM and never higher than 10 µM. Well known products like Cisplatine, Doxorubicine, 5-Fluoruracile evaluated in MCF-7 breast carcinoma cells line, the Placliex, Taxol and Placlitaxel assessed in ten different tumor cell lines and Vincristina, Placlitaxel in four cervical carcinoma cell lines26-28 all of them were always quite effective at very low doses under 10µM and showed to be specific by tumor cell lines.
Contrary to the previous results mentioned before, the IC50value obtained with these compounds in both cell lines suggests that the addition of another bromine atom (at C-2 position of the lateral carbon chain outside the furan ring) in the G1 product caused a loss of cytotoxic activity, maybe because the interactions of halogen bounds between the two bromine atoms on the test substance affect the direct halogenation of some target molecules no yet identified.
One of the most important properties of the product to be selected as antitumoral drug is the mitotic index value. The MI variable obtained at in vitro cell culture experiments is used by several authors as a measure of efficiency of the test substance29-31. We believe that the lowest toxicity of G1 with respect to MA arises from their different geometries. If these two molecular species interact directly with their biological substrates, not yet identified, two different types of complexes are expected for, one less active than the other one. Or perhaps, in the G1 solution, molecules of G1 interact between them more efficient, forming complexes by means of halogen bounds between two Br atoms and the nitro group of the lateral carbon chain. The formation of these G1 complexes can reduce the effectiveness of this substance on the target molecules, rendering it less effective than MA.
This work is the first in vitro cytotoxicity report comparing the efficacy of 2 furylethylene derivatives with different numbers of bromine atoms on cell proliferation. Other experiments must be performed to establish the structure activity relationships (SAR) of these 2-furylethylene derivatives over this molecules activity.
Now a plausible explanation cannot be offered for such a difference between G1 and MA products but nonetheless, this work serves as a warning that intermolecular forces such as hydrogen bonds, salt bridges and van der Waal's forces are critical determinants of biological structure and function. For that reason, it is not surprising that intense efforts had been dedicated to the understanding of how these forces contribute to enzymatic reactions, protein folding, and ligand binding; in order to use them more effectively to control biological recognition.
CONCLUSIONS
The presence of two bromine atoms on the molecular structure of 2 furylethylene derivative decreased de cytotoxic effect of these products evidenced by de results obtained with the G1 composite in both cell lines. Nevertheless neither MA nor G1 showed specificity for tumoral cells with IC50higher than 100 µM.
The compound tested (MA) was classified to 3th toxicity class with fixed LD50 cut off value 200 mg/kg.
ACKNOWLEDGEMENTS
We want to thank Dr. Ana M. Martínez Campos by her contribution with the pathologic analysis and the professors Ida Padilla and Aniorland García Borrego for reviewing the manuscript.
CONFLICT OF INTEREST
The authors of this paper declare that there are not conflicts of interests.
REFERENCES
1.- 1. Estrada E. Structure-mutagenicity relationships in 2-furylethylene derivatives. A molecular orbital study of the role of nitro groups. Mutat Res. 1998;420:67-75.
2.- González-Díaz H, Olazábal E, Santana L, Uriarte E, González-Díaz Y, Castañedo N. QSAR study of anticoccidial activity for diverse chemical compounds: Prediction and experimental assay of trans-2-(2-nitrovinyl)furan. Bioorg Med Chem. 2007;15:962-968.
3.- Castañedo N, Goizueta R, Perez J, Gonzalez J, Silveira E, Cuesta M, et al. , inventors. Procedure for the obtainment of 1-(5- bromofur-2-il)-2-bromo-2-nitroethene and its microcide action. Cuba 1994.
4.- Hulbert P, Bueding E, Robinson C. Structure and antischistosomal activity in the nitrofuryl series. J Med Chem 1973;16:72-78.
5.- Yahagi T, Nagao N, Hara K, Matsushima T, Sugimura T, Bryan T. Relationships between the carcinogenic and mutagenic or DNA modifying effects of nitrofuran derivatives, including 2 2-furyl.-3 5-nitro-furyl.acrylamide, a food additive. Cancer Res 1974;34:2266-2273.
6.- McCalla D. Mutagenicity of nitrofuran derivatives. Environ Mutagen 1983;5:745-65.
7.- Sturdik E, Drobnica L, Balaz S. Interactions of 2-furylethylenes with thiol enzimes. Coll Czech Chem Commun 1983;48:327-335.
8.- Rosenberg M, Balaz S, Sturdik E, Kucha´r A. Reactivity of 2-furylethylenes with nucleophilic groups and its biological significance. Coll Czech Chem Commun 1987;52:425-36.
9.- Hertiani T, Edrada-Ebel R, Ortlepp S, van Soest RW, de Voogd NJ, Wray V, et al. From anti-fouling to biofilm inhibition: new cytotoxic secondary metabolites from two Indonesian Agelas sponges. Bioorg Med Chem. 2010;18:1297-1311.
10.- Moubax I, Bontemps-Subielos N, Banaigs B, Combaut G, Huitorel P, Girard JP, et al. Structure-activity relationship for bromoindole carbaldehydes: effects on the sea urchin embryo cell cycle. Environ Toxicol Chem. 2001;20:589-596.
11.- Carletti I, Banaigs B, Amade P. Matemone, a new bioactive bromine-containing oxindole alkaloid from the indian ocean sponge Iotrochota purpurea. J Nat Prod. 2000;63:981-983.
12.- Serra AC, Pineiro M, Rocha Gonsalves AM, Abrantes M, Laranjo M, Santos AC, et al. Halogen atom effect on photophysical and photodynamic characteristics of derivatives of 5,10,15,20-tetrakis(3-hydroxyphenyl)porphyrin. J Photochem Photobiol B. 2008;92:59-65.
13.- Jiang Y, Lin HX, Li M, Wu BL, Chen JM. Preparation and evaluation of new brominated paclitaxel analogues. J Asian Nat Prod Res. 2005;7:231-236.
14.- Sisa M, Pla D, Altuna M, Francesch A, Cuevas C, Albericio F, et al. Total synthesis and antiproliferative activity screening of (+/-)-aplicyanins A, B and E and related analogues. J Med Chem. 2009;52:6217-6223.
15.- Hladon B, Goslinski T, Laskowska H, Baranowski D, Ostrowski T, Zeidler J, et al. In vitro cytostatic activity of 8-substituted and tricyclic analogues of acyclovir. Pol J Pharmacol. 2002;54:45-53.
16.- Henderson JP, Byun J, Williams MV, McCormick ML, Parks WC, Ridnour LA, et al. Bromination of deoxycytidine by eosinophil peroxidase: a mechanism for mutagenesis by oxidative damage of nucleotide precursors. Proc Natl Acad Sci U S A. 2001;98:1631-1636.
17.- Henderson JP, Byun J, Williams MV, Mueller DM, McCormick ML, Heinecke JW. Production of brominating intermediates by myeloperoxidase. A transhalogenation pathway for generating mutagenic nucleobases during inflammation. J Biol Chem. 2001 Mar 16;276:7867-7875.
18.- Kraut D, Churchill M, Dawson P, Herschlag D. Evaluating the potential for halogen bonding in the oxyanion hole of ketosteroid isomerase using unnatural amino acid mutagenesis. ACS Chemical Biology. 2009;4:269-273.
19. Auffinger P, Hays FA, Westhof E, Ho PS. Halogen bonds in biological molecules. Proc Natl Acad Sci U S A. 2004;101(48):16789-16794.
20.- Rahman A, Iqbal M, Thomsen W. Bioassay Techniques for Drug Development. Harward Academia. 2001.
21.- Dierickx P. Cytotoxic of the MEIC reference chemicals in rat hematoma derived Fa32 cells. Toxicology. 2000;150:159-69.
22.- Barbini L, Lopez P, Ruffa J, Martino V, Ferraro G, Campos R, et al. Induction of apoptosis on human hepatocarcinoma cell lines by an alkyl resorcinol isolated from Lithraea molleoides. World J Gastroenterol 2006;12:5959-5963.
23.- Verma R, Babu A (eds). Human Chromosomes: Principles and Techniques, 2nd edn. McGraw-Hill: New York. 1995. 9-29.
24.- Martínez Y. Actividad citotóxica de derivados del 2-furiletileno en líneas celulares normales y neoplásicas. Thesis [Pharmacology Specialist]. Camaguey, Cuba: Medical Sciences University; 2010.
25.- Rahden-Staron I, Czeczot H, Szumilo M. Induction of rat liver cytochrome P450 isoenzymes CYP 1A and CYP 2B by different fungicides, nitrofurans, and quercetin. Mutat Res. 2001;498:57-66.
26.- Lopez Lopez R, van Rijswijk RE, Wagstaff J, Pinedo HM, Peters GJ. The synergistic and antagonistic effects of cytotoxic and biological agents on the in vitro antitumour effects of suramin. Eur J Cancer. 1994;30A(10):1545-1549.
27.- Hassan S, Dhar S, Sandström, Arsenau D, Budnikova M, Lokot I, et al. Cytotoxic activity of new paclitaxel formulation, Pacliex, in vitro and in vivo. Cancer Chemother Pharmacol 2005;55:47- 54.
28.- Juang S, Lung Ch, Hsu P, Hsu K, Li Y, P. H. D-501036, a novel selnophone- based triheterocycle derivate, exhibits potent in vitro and in vivo antitumoral activity which involves DNA damage and ataxia telangiectasia- mutated nuclear protein kinase activation.. Mol CancerTher 2007;6(1):193-202.
29.- Wiebe JP, Beausoleil M, Zhang G, Cialacu V. Opposing actions of the progesterone metabolites, 5alpha-dihydroprogesterone (5alphaP) and 3alpha-dihydroprogesterone (3alphaHP) on mitosis, apoptosis, and expression of Bcl-2, Bax and p21 in human breast cell lines. J Steroid Biochem Mol Biol. 2010;118(1-2):125-132.
30.- Soldati R, Wargon V, Cerliani JP, Giulianelli S, Vanzulli SI, Gorostiaga MA, et al. Inhibition of mammary tumor growth by estrogens: is there a specific role for estrogen receptors alpha and beta? Breast Cancer Res Treat. 2010;123(3):709-724.
31.- Smilek P. [Molecular predictors in head and neck tumours]. Klin Onkol. 2010;23(4):218-23.
CORRESPONDENCE:
Oscar Hernández Betancourt
Centro de Inmunología y Productos Biológicos (CENIPBI),
Universidad Médica.
Camaguey.
CP 70100. Apdo 150.
Cuba
oscar.hernandez1964 @ yahoo.es
