Indice del volumen Volume index
Comité Editorial Editorial Board
Comité Científico Scientific Committee
A LIGHT AND SCANNING ELECTRON MICROSCOPY STUDY OF PLACENTAL VILLI ASSOCIATED WITH OBESITY AND HYPERTENSIÓN
Olivar Clemente Castejón Sandoval y Angela Josmar López González
Laboratory of Electron Microscopy. Center for Research and
Analysis Assistancel Teaching of the Nucleus Aragua (CIADANA).
Faculty of Health Sciences. University of Carabobo
Aragua State. Maracay. Venezuela.
olivar.ciadanauc @ gmail.com
Rev Electron Biomed / Electron J Biomed 2013;2:29-36.
RESUMEN:
Objetivo: El propósito de este estudio fue examinar la estructura de la vellosidad placentaria asociada con obesidad e hipertensión usando microscopia de luz y de barrido.
Metodos: Dos placentas a termino de mujeres embarazadas asociadas a nacidos muertos fueron tomadas para análisis microscópico. Embarazadas pesaron 75 y 85kg y su hipertensión con mas de 90-150mm Hg. Edades de 39 y 41 respectivamente fueron y pesos placentarios de 600 y 650 Vellosidadesgr después de drenar la sangre durante 30 minutos post parto. Cinco fragmentos fueron tomados por cada placenta y evaluada con microscopis de luz y de barrido.
Resultados: Vellosidades troncales mostraron vasos obstruidos con daños en las paredes. Tejido reticular bajo el sincitio fue un hallazgo permanente. Abundan las vellosidades intermedias inmaduras. Cambios degenerativos severos se notan en la periferia de troncales. Vellosidades fibroticas, intermedias maduras pobremente desarrolladas, terminales filiformes, corangiosis, deposición de fibrinoide y vasodilatación fueron encontradas. No se observa inflamación y arborización fue vista muy escasa.
Conclusiones: Estos resultados indican inmadurez persistente, bajo grado de maduración y cambios degenerativos afectando la estructura de la vellosidad sin inflamación contribuyendo probablemente con la muerte fetal.
PALABRAS CLAVES: Microscopio de luz. Microscopio Electrónico de Barrido. Vellosidad placentaria. Obesidad. Hipertension.
SUMMARY:
The aim of this study was to examine the structure of the placental villi associated with obesity and hypertension using light and scanning electron microscopy.Two placentas at term obtained of woman pregnancy associated to stillborn were taken for microscopical analysis.
Woman pregnancy weighed 75 and 85 Kg as body mass index and their hypertension with more of 90/150 mmHg. They had 39 and 41 years old. The placental weights were 600 and 650 gr respectively after of draining all their blood during 30 minutes post delivery.
Five small fragments were taken by each placenta and evaluated with light and scanning electron microscopy. Stem villi showed obstructive vessels with damage in their walls and reticular tissue under the syncytio was a permanent finding. Immature intermediate villi were frequent. Severe degenerative changes are noted in peripheric stem villi. Fibrotic villi, very poor developed mature intermediate villi, filiform terminal villi, corangiosis, deposition of fibrinoid and vasodilatation were found in placental villi. No inflammation and low arborization was seen.
These results indicate immaturity persistent, low maturation degree and severe degenerative changes affecting the structure of placental villi without inflammation.
KEYWORDS: Light and Scanning Electron Microscopy. Placental villi. Obesity Hypertension.
INTRODUCTION
Maternal obesity is a frequent obstetric risk factor, linked with short and long term consequences for mother and child, including fetal overgrowth and stillbirth 1. It is associated with increased risk of hypertension. This complication of pregnancy also has been linked with increased incidence of fetal intrauterine growth restriction and pre-eclampsia 2.
In a non human primate model of excess nutrition there was a statistically significant reduction in uterine volume blood flow and increased frequency of infarctions, calcifications and syncytial knothing. In this study was observed that maternal high-fat diet disturbs utero placental hemodinamics and increases the frequency of stillbirth3.
It is known that in middle-aged and older woman pulse wave velocity increased with higher body mass index2. Increased placental weight and placental hypertrophy have been more common in obese groups4. Normal placental weights have increased over the last decades and this may correlate with increasing maternal obesity5.
Fetal overgrowth provoking macrosomia and lipid overload is observed in maternal over nutrition and obesity2,6-7 which increased newborn cholesterol that could modify the fetus metabolism and its predisposition to develop diseases later in life8.
Maternal obesity is associated with a maternal inflammatory state that induces structural and functional changes in the placenta. Increased placental and adipose tissue macrophage infiltration has been found9-11. The placenta in the hostile inflammatory environment of maternal obesity could be accompanied by alterations as endothelial function impaired which would be associated with prothrombotic state or coagulation factors11. This inflammatory milieu may have critical consecuences to the fetus in their future live12.
Maternal obesity may also affect placental transport and substrate availability. Adipose tissue as an endocrine organ generate leptin and adiponectin, adipokines expressed and secreted during pregnancy associated with obesity that influence the angiogenesis and the endothelial function of the placental villi. Given that maternal obesity is accompanied by significant dysregulation of normal physiology, it is therefore plausible that placental structure and function may be altered as a consequence of maternal obesity and equally that placenta may modulate maternal physiology by release of inflammatory cytokines11. The mechanisms underlying the influence of adiposity and hypertension on the properties of the placental structure are unknown and morphological studies are required. Adaptations of the placenta in response to obesity are limited and contradictory results are available in the literature.
Taking into account the aforesaid considerations, this paper reports the observations of placental tissue associated with obesity and hypertension using light and scanning electron microscopy.
CASES REPORT
Two placentas obtained of woman pregnancy at 38 weeks of gestation and associated to stillborn were taken for microscopical analysis. The patients of 39 and 41 years old were strictly informed following basic ethical principles of the declaration of Helsinki as well as the rules of the Ethical Committee of our institution.
Obesity is defined as body mass index (BMI) ? 30 Kg/m2. Hypertension is defined as blood pressure > 90/150 mmHg. Woman pregnancy weighed 75 and 85 kg as body mass index and their hypertension with more of 90/150 mmHg. The placental weights were 600 and 650 gr after of draining all their blood during 30 minutes immediately post delivery.
This study is descriptive, retrospective and no experimental with no probabilistic sampling. The observations found in placental villi were selected in two groups: a group study and control group. A protocol of features was applied to these two groups determining structural characteristics of placental villi associated with obesity using light and scanning electron microscopy (SEM). Cross sections of placental villi stained with H-E will be associated with similar regions taken with SEM.
Light microscopy (LM): five biopsy by each placenta were taken and of each one three slides were prepared for H-E staining. These were compared with normal slides. For evaluate the microscopical lesions 20 camps by slide were seen in a standard clinical Zeiss microscope with the 12x objective. Immaturity was defined in the term placenta by the incidence of the large villi with an abundance of stroma and relatively small fetal vessels which are positioned centrally rather than peripherally. Edema villous is defined as hydropic appearing of empty spaces under plasmamembrane of the syncytio. Fibrinoid deposition as the presence of fibrin plaques into heterogenous material still unknown that can contain x-trophoblasts cells.Corangiosis as ten o more capillaries in ten o more villi in ten o more microscopic fields at x100 magnification13.
Scanning Electron Microscopy (SEM): five small fragments from the basal plate were taken for SEM according to conventional stains and the results obtained were compared with normal observations and seen with a Hitachi S2300 scanning electron microscope. The nomenclature here described of the placental villi is the used by Benirschke and Kaufmann13: stem villi (sv), mature intermediate villi (miv), immature intermediate villi (iiv) and terminal villi (tv).Classification described by them based on their histological features.
RESULTS
Peripheric stem villi observed in the intervillous space showed obstructive vessels while those others were seen with damage in the endothelial layer. Numerous of them presented occlusive or dilated vessels congestioned by erythrocytes. No thrombosis was observed in these vessels but a hyperplasia of muscular media was noted in the main stems of the villous tree.
Images as observed in Fig 1a were dominant in the observations with SEM and correspond with immature intermediate villi. Cross sections of immature placental villi are seen with LM in Fig 1b.
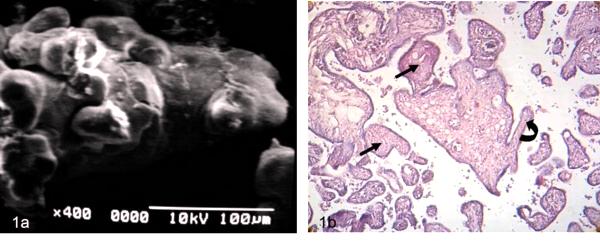
Figure 1 a) Immature intermediate villi showing globulous regions in their periphery SEM.
b) Cross sections of two immature intermediate villi are seen. The arrows indicate fibrotic villi. Curve arrow signals mature intermediate villi. 120X H-E.
The cell surface of the syncytiotrophoblast showed interruptions and a plasma membrane very thin in some zones, regions that lacks of nucleus and subtrophoblastic edema were noted.
Dilated blood vessels overcrowded of erythrocytes were found in the observations. Sometimes the stromal region is separated of the syncytio. Necrosis of the synytiotrophoblast is observed with frequency. So, zones of hipercapillarization or corangiosis also were observed. Vasodilatation was noted in the vessels of stem villi with frequency (Figs 2a, b).
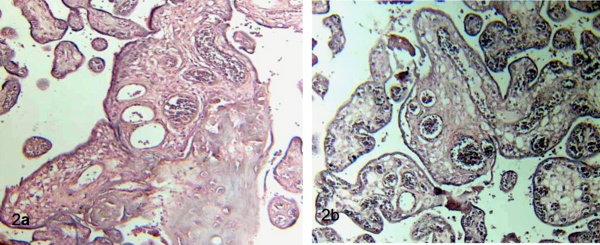
Figure 2 a) Stem villi shows thinning of the syncytio and interruptions, zones of necrosis and
strong deposition of matrix-type fibrinoid. Terminal villi surrounding stem villi are seen. 120 X. H-E.
b) Placental villi with corangiosis or hipercapillarization stromal are noted. In the central stem villi subtrophoblastic edema is notorious. 120 X. H-E.
Peripheric and more terminal branches of the villous tree were seen as very fibrotic when seen with LM (Figs 3a, b).
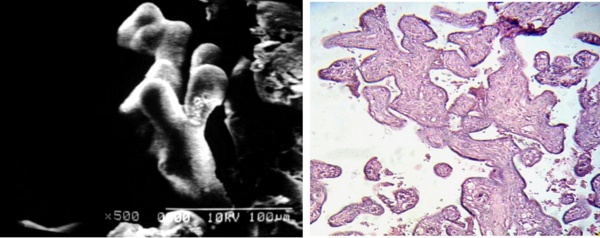
Fig 3 a) Small trayectory of terminal branches of peripheric stem villi are observed with shorter dicotomic ramifications SEM.
b) Cross sections of placental villi showing a dense fibrotic stroma. 120 X. H-E.
Some of these stem villi are long with right course and with very poor developed mature intermediate villi and blood vessels emptied of erythrocytes in any cases (Figs 4a, b).
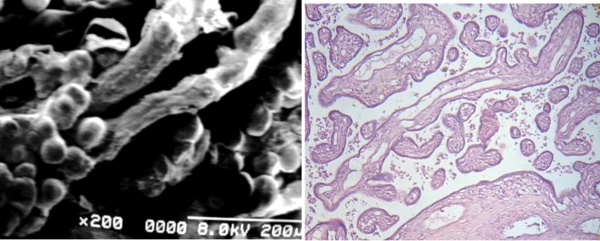
Fig 4 a) Two small stem villi affected by Fibrin-type fibrinoid associated to immature intermediate villi with globulous extensions. SEM.
b) Cross sections of stem villi (arrows) exhibing emptied vessels.
The stem villi crossing the microphotograph shows incompletes mature intermediate villi.
Part of a section of large stem villi with reticular tissue in their periphery with damaged vessels is seen under to the right. 120X. H-E.
Filiform terminal villi were noted in groups associated with placental villi of different thickness and morphology (Figs 5a, b).
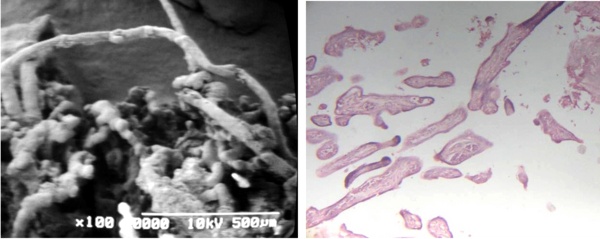
Fig 5 a) Filiforms terminal villi are observed in association with maldeveloped villi in contact with Fibrin-type fibrinoid SEM.
b) Cross sections of filiform terminal villi are seen. 120 X. H-E.
Regions of placental villi conforms zones of pre-infarctions (Figs 6a, b), where a mixing of mature and immature villi are found. The plasma membrane of the syncytiotrophoblast presents numerous globulous or ovals protrusions of different size when the surface of these villi are seen with SEM (Figs 7a, b).
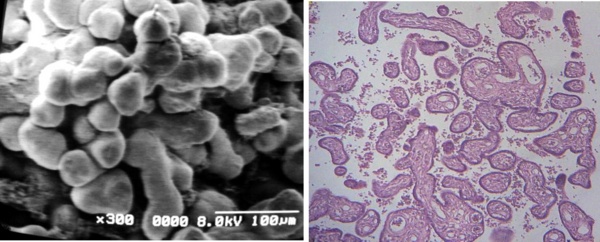
Fig 6 a) Region of a conglomerate of mature and immature villi associating as zone of pre-infarcts SEM.
b) Cross sections of mature intermediate villi (arrow) and immature intermediate villi (curve arrow).
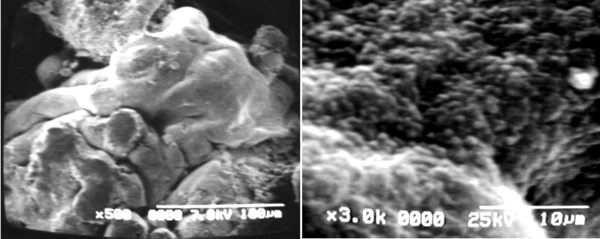
Fig 7 a) Immature intermediate villi extending three mature intermediate villi to the left of the microphotografh SEM
b) Cell surface of placental villi showing details of microvilli of the syncytiotrophoblast SEM.
Many placental villi presented a strong deposition of fibrinoid. This deposition can be surrounding the surface of the villi (Fig 8a) or to be in stromal regions as seen in Fig 8b. The deposition of fibrinoid was a characteristic feature of the sampling realized. The presence of numerous stem villi with reticular tissue under the syncytiotrophoblast was a permanent finding in these observations. These stem villi have not obtained their total development. In these observations no inflammation or presence of inflammatories cells as macrophages or neutrophils were not seen.
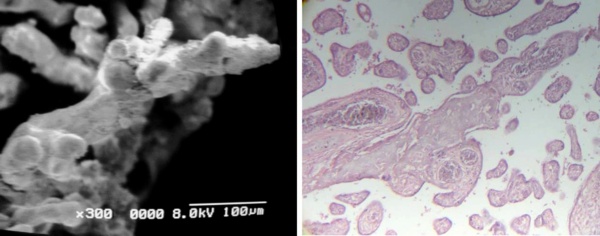
Fig 8 a) Peripheric stem villi associated with Fibrin-type fibrinoid. SEM.
b) Cross sections of peripheric stem villi with stromal fibrinoid separated of the syncytiotrophoblast. 120 X. H-E
DISCUSSION
In this paper is shown that obesity associate with hypertension produce severe degenerative changes in stem villi and other placental villi. Although no thrombosis was observed in stem villi, it could to be possible that obstructive lumina of the vessels in the chorionic plate leads to villous stromal changes in the placental villi or in the syncytio by reduction of blood flow.Hypertension appears to induce vascular endothelial damage.
Maternal hypertension predispose to decresead uteroplacental blood flow which could to stimulate few attracting of blood by intermediate placental villi provoking isquemic placental villi with degenerative changes.The underperfusion of the intervillous space has resulted in local areas with increased perivillous fibrin and villous agglutination. Growing evidence in human and animal models of maternal obesity indicate several placental changes: increased idiopathic villitis, macrophage infiltration and placental vascularity9.
A reduction of blood flow in the intervillous space results in degenerative changes as the here observed. Others studies report that are not there changes a level ultrastructural2 or structural14. Roberts et al10 not found difference in edema in placenta from obese compared to non-obese woman. Our results permit to observe regions of stroma separated of the syncytio by edema.
The presence of numerous stem villi with reticular tissue under the syncytio is indicative of a placenta that is in the 18th week suffering of persistent immaturity.
A low arborization showed by an abundance of immature intermediate villi as seen in the results testify a low placental maturity being expression of a low maturation15; although there was no difference in placental maturity in placental from obese compared to non-obese woman10. It is possible that the differences may be due to the considered patient. We no excluded women with gestational hypertension but in the work previously mentioned10 gestational hypertension was excluded.
The features of the syncytio here found are demonstrative of that during obesity associated with hypertension this tissue can be suffering severe degenerative changes with dysfunction of the protective barrier to the fetus. This is in contrast to previously published findings in which villous and syncytiotrophoblast area and total nuclear number remain unaffected14 although reduced placental proliferation described by them and Higgins et al.1 could be accelerated by this cause. This possibility is evidenced by terminal and more slender branches of the villous tree here seen as very fibrotic.
The observation of stem villi with some emptied vessels of erythrocytes indicate that fetus can to have problems in to absorb gases and nutrients or that placental villi are with bad perfusion promoting an ambient of hyperoxia which brake the process of braching angiogenesis resulting in filiform terminal villi16. The ultrasonographic diagnostic of these elements in increased quantity has been enough for immediate termination of the pregnancy17. These villi of minimum caliber are atrophied by extensive hypoxic villous damage produced probably by low blood flow in the intervillous space3 due to obstructive or damaged vessels of stem villi in the chorionic plate.
Microvilli of the syncytiotrophoblast have been observed reduced in number15, slender and thicker in their extremity when seen with transmission electron microscopy in the baboon9; in contrast with the noted by us as irregular or globulous extensions that probably permit a higher nutrient uptake.
It has been hypothesized that maternal obesity results in increased placental nutrient transport to the fetus18-19.
The extent of lipid transfer to the fetus strongly contributes to fetal fat accretion. Human in vivo studies using labeled fatty acids reported a preferential placental-fetal transfer of long-chain poly insatured fatty although the mechanisms are still uncertain. Knowledge about fatty acids metabolism and adaptations of the placenta in response to obesity are more limited and contradictory results are available in the literature7-8.
So decreased, placental amino acid transporter activity was associated with maternal obesity20 while that in microvillous plasma membrane isolated from placentas of high-fat diet-fed animals there was increased aminoacid transporters9.
It has been described that reduced placental taurine transporter activity in pregnancies complicated by pre-eclampsia and maternal obesity could contribute dysregulated renewal of syncytiotrophoblast since taurine is a important nutrient for fetal organ development and cellular removal of this tissue21.
Blood vessel structure is altered in obesity with increase in vessel diameter22, limiting caliber and distensibility of vessel walls are mechanisms that could affect placental blood vessel structure in maternal obesity. Enhanced understanding of normal and aberrant placental structure of vessel in early pregnancy of obese woman is required23.
Degenerative changes as seen in Figs 2a, b justify the presence of matrix-type fibrinoid and fibrin-type-fibrinoid that could to be involved besides in maternofetal transport processes as mentioned above. Where the thinner syncytio is interrupted by degeneration or by mechanical forces, the gap is immediately filled by fibrin-type fibrinoid resulting from the coagulation cascade13.
These fibrinoid spots provide an effective transfer route for macromolecules or gases in the maternofetal interchange. Hipercapillarization of these villi is an adaptative response of the placenta to the reduction of blood flow in the intervillous space. A progressive decrease in total lymphocyte and monocyte count which occurs during pregnancy before term could to explain of this manner the absence of inflammatories cells in this work24. On the other hand, an exhaustive control of urinary infection could to have existed.
CONCLUSION
In conclusion, severe degenerative changes affecting the structure of placental villi are seen in placentas associated with obesity and hypertension, which presented immaturity persistent, without inflammation or infiltration by macrophages and neutrophils with a low arborization indicating low maduration degree.
REFERENCES
1) Higgins L, Mills TA, Greenwood SL, Cowley EJ, Sibley CP, Jones RL. Maternal obesity and its effect on placental cell turnover. Journal of Maternal & Fetal Neonatal Medicine. 2013;26:1-6.
2.- Samson JE, Mari G, Dick EJ Jr, Hubbard GB, Ferry RJ Jr, Schlabritz-Loutsevitch NE. The morphometry of materno-fetal oxygen exchange barrier in a baboon model of obesity. Placenta. 2011;32:845-851.
3.- Frias AE, Morgan TK, Evans AE, Rasanen J, Oh KY, Thornburg KL, et al. Maternal high-fat diet disturbs uteroplacental hemodynamics and increases the frequency of stillbirth in a nonhuman primate model of excess nutrition. Endocrinology. 2011;152:2456-2464.
4.- Wallace JM, Horgan GW, Bhattacharya S. Placental weight and efficiency in relation to maternal body mass index and the risk of pregnancy complications in women delivering singleton babies. Placenta. 2012;8:611-618.
5.- Swanson LD, Bewtra C. Increase in normal placental weights related to increase in maternal body mass index.Journal of Maternal & Fetal Neonatal Medicine. 2008;21:111-113.
6.- Merzouk H, Meghelli-Bouchenak M, Loukidi B, Prost J, Belleville J. Impaired serum lipids and lipoproteins in fetal macrosomia related to maternal obesity. Biology of the neonate. 2000;77;17-24.
7.- Gil-Sánchez A, Koletzko B, Larqué E. Current understanding of placental fatty acid transport. Current Opinion in Clinical Nutrition and Metabolic Care. 2012;15:265-272.
8.- Dubé E, Gravel A, Martin C, Desparois G, Moussa I, Ethier-Chiasson M, et al. Modulation of Fatty Acid Transport and Metabolism by Obesity in the Human Full-Term. Biology of the Reproduction. 2012; 87:1-11.
9.- Farley D, Tejero ME, Comuzzie AG, Higgins PB, Cox L, Werner SL, et al. Feto-placental adaptations to maternal obesity in the baboon. Placenta. 2009;30:752-760.
10.- Roberts KA, Riley SC, Reynolds RM, Barr S, Evans M, Statham A, et al. Placental structure and inflammation in pregnancies associated with obesity. Placenta. 2011;32:247-254.
11.- Denison FC, Roberts KA, Barr SM, Norman JE. Obesity, pregnancy, inflammation, and vascular function. Reproduction.2010;140:.373-378.
12.- Challier JC, Basu S, Bintein T, Minium J, Hotmire K, Catalano PM, et al. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta. 2008;29:274-281.
13.- Benirschke K, Kaufmann P. Pathology of the human placenta. 4th edition. New York: Springer-Verlag; 2000.
14.- Higgins L, Mils TA, Greenwood SL, Wareing M, Jones RL, Courley EJ. et al., Maternal Obesity: does placenta cell turnover hold clues the aetiology of aberrant fetal growth?. Reproduction Science. 2010;17: S453.
15.- Biagini G, Vasi V, Pugnaloni A, Valensise H, Rizzoli R, Miccoli MC, et al., Morphological development of the human placenta in normal and complicated gestation: a quantitative and ultrastructural study. Gynecologic and Obstetrics Investigation. 1989;28: 62-69.
16.- Kingdom J, Huppertz B, Seaward G, Kaufmann P. Development of the placental villous tree and its consequences for fetal growth. European J ournal of Obstetrics, Gynecology, and Reproductive Biology. 2000;92:.35-43.
17.- Hargitai B, Marton T, Cox PM. Examination of the human placenta. Journal of Clinical Pathology. 2004;57: 785-792.
18.- Jones HN, Powell TL, Jansson T. Regulation of Placental Nutrient Transport - a review . Placenta. 2007;28:763-774.
19.- Jones HN, Woollett LA, Barbour N, Prasad PD, Powell TL, Jansson T. High-fat diet before and during pregnancy causes marked up-regulation of placental nutrient transport and fetal overgrowth in C57/BL6 mice. FASEB J. 2009; 23: 271-278.
20.- Farley DM, Choi J, Dudley DJ, Li C, Jenkins SL, Myatt L, et al., Placental amino acid transport and placental leptin resistance in pregnancies complicated by maternal obesity. Placenta. 2010; 31: 718-724.
21.- Desforges M, Ditchfield A, Hirst CR, Pegorie C, Martyn-Smith K, Sibley CP, et al., Reduced Placental Taurine Transporter (TauT) Activity in Pregnancies Complicated by Pre-eclampsia and Maternal Obesity. Advances in Experimental Medicine and Biology. 2013;776:81-91.
22.- Zebekakis PE, Nawrot T, Thijs L, Balkestein EJ, van der Heijden-Spek J, Van Bortel, et al.,Obesity is associated with increased arterial stiffness from adolescence until old age. Journal of Hypertension. 2005;23:1839-1846.
23.- Higgins L, Greenwood SL, Wareing M, Sibley CP, Mills TA. Obesity and the placenta: A consideration of nutrient exchange mechanisms in relation to aberrant fetal growth. Placenta. 2011;32:1-7.
24.- Valdimarsson H, Mulholand C, Fridriksdottier V, Coleman DV. A longitudinal study of leucocyte blood counts and lymphocyte responses in pregnancy; A marked early increase of monocyte-lymphocyte ratio. Clinical and Experimental Immunology. 1983;53:437-443.
ACKNOWLEDGMENTS
We are deeply grateful to the delivery room staff at the Maracay Central Hospital for their help in obtaining placenta, and to the administrative coordination of the Health Sciences Faculty of the Carabobo University of Nucleus Aragua Venezuela by financial support for CIADANA.
CORRESPONDENCE:
Prof. Olivar C Castejón.
General Coordinator of the CIADANA. Professor in Cell Biology.
Faculty of Health Sciences.
University of Carabobo -
Aragua State. Maracay. Venezuela.
Apdo. 4944.
olivar.ciadanauc @ gmail.com
Received: November 27, 2013.
Published: December 30, 2013.
