Indice del volumen Volume index
Comité Editorial Editorial Board
Comité Científico Scientific Committee
INDOLEAMINE 2,3-DIOXYGENASE (IDO) AND IMMUNE TOLERANCE
María Jesús Coma-del-Corral1, Pilar Muñiz Rodríguez2,
Joaquín Terán Santos3
1Unidad de Investigación del Hospital Universitario de Burgos.
2Departamento de Biología y Bioquímica molecular de la Universidad de Burgos
3Unidad de Sueño del Hospital Universitario de Burgos.
Burgos. España
mjcoma @ hubu.es
Rev Electron Biomed / Electron J Biomed 2013;3:53-58.-
Comment of the reviewer Paula A. Enz MD. Chief of Cutaneous Lymphomas, Photopheresis, and Phototherapy Section. Dermatology Division. Hospital Italiano de Buenos Aires - Argentina.
Comentario del revisor Dr Carlos G. Musso. Carlos G. Musso, MD. (UBA), PhD. (U. Salamanca). IAGG Research Network. Sub-Chief Nephrology and Internal Milieu Division
SUMMARY:
Indoleamine 2,3-dioxygenase (IDO) is an intracellular and extrahepatic enzyme predominantly found in many cells, especially macrophages. Tryptophan degradation generates kynurenine, and this pathway of tryptophan metabolism is an effective mechanism for modulating the immune response.
The IDO facilitates immune tolerance and is one of the main actors involved in the inhibition of cell proliferation, including activated T cells. IDO induces production of reactive oxygen species (ROS) and nitric oxide (NO) radicals. Several pathways involved in the regulation of immune response are regulated by redox mechanisms. Reactive oxygen and nitrogen species (ROS-RNS) and other redox active molecules play key roles in immunity.
KEYWORDS: Indoleamine 2,3-dioxygenase. Tryptophan metabolism. Kynurenine
RESUMEN:
La indolamina 2,3-dioxigenasa (IDO) es un enzima predominante extrahepática intracelular y se encuentra en numerosas células, principalmente macrófagos. La degradación del triptófano genera quinurenina, y esta vía del metabolismo del triptófano, constituye un mecanismo eficaz de modulación de la respuesta inmune.
La IDO facilita la tolerancia inmunológica, y es uno de los principales actores implicados en la inhibición de la proliferación celular, incluyendo las células T activadas. La IDO induce la producción de especies reactivas de oxígeno (ROS) y radicales de óxido nítrico (NO). Varias vías implicadas en la regulación de respuesta inmunológica se regulan por mecanismos redox. Las especies reactivas del oxígeno y nitrógeno (ROS-RNS) y otras moléculas activas redox cumplen funciones clave en la inmunidad.
PALABRAS CLAVES: Indolamina 2,3-dioxigenasa. Metabolismo del Triptófano. Quinurenina
Tryptophan and kynurenine
There are nine amino acids that are called "essential" because the body is unable to synthetize them and must acquire them from external sources, mainly through the diet. One of these is tryptophan. Once it has been absorbed in the body, it can be found in the circulation in the free form or, most commonly, bound to albumin. Tryptophan plays a role in protein synthesis, as well as in several metabolic pathways, producing different metabolites, of which kynurenine is one1.
Tryptophan can be metabolized via different pathways. It is oxidized by opening the indole ring, which is started either by the enzyme tryptophan 2,3-dioxygenase (TDO), which is mainly found in the liver, and is induced by corticosteroids2, or by another enzyme called indoleamine 2,3-dioxygenase (IDO)3.
Indoleamine 2,3-dioxygenase (IDO) is predominantly an extrahepatic enzyme, and can be found in numerous cells, including macrophages, microglia, neurons and astrocytes 4-6. Kynurenine is produced during the metabolism of tryptophan. This pathway is the most important route of tryptophan metabolism, since it is an efficient modulation mechanism of the immune response. Furthermore, other metabolites from this pathway can synergize or antagonize these effects 3. ]. This pathway is regulated by certain cytokines and inflammatory molecules, among which is interferon gamma (IFN-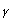 7 which induces IDO in cells of the immune system 8-9. IDO is effectively the first enzyme of that pathway, and is activated when an immune response is produced via IFN-
7 which induces IDO in cells of the immune system 8-9. IDO is effectively the first enzyme of that pathway, and is activated when an immune response is produced via IFN-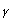 . In turn, the depletion of tryptophan and the production of kynurenine modulate the immune response.
. In turn, the depletion of tryptophan and the production of kynurenine modulate the immune response.
The kynurenine pathway is implicated in many diseases and disorders, and there are many pathological conditions in which an imbalance between tryptophan and kynurenine has been found. The list, up until now, includes neoplastic diseases, protozoal infections such as malaria, as well as bacterial and viral infections, such as Human Immunodeficiency Virus (HIV), autoimmune diseases such as rheumatoid arthritis and multiple sclerosis, and a wide spectrum of neurological conditions such as Alzheimer's disease, lateral amyotrophic sclerosis, and Huntington's chorea, as well as psychiatric diseases such as depression and schizophrenia. We can see a panorama:
IDO possesses an effective bactericide mechanism and has been associated with cell immunosuppression. IDO activity in infected cells provokes a powerful bactericide effect to fight against the propagation of infection by means of the degradation of tryptophan by autotrophic bacteria, causing their death. The metabolites, among which is kynurenine, also have a bactericide effect that is toxic for bacteria. In the last few years, increasing evidence has been shown that IDO also plays an important role in viral infections, including HIV 10, hepatitis B and C 11, and influenza12.
In patients on hemodialysis, there is an increase in tryptophan degradation associated with an increase in the concentration of neopterin, which indicates the active involvement of IDO13.
Some neuropsychiatric symptoms that appear in people of advanced age seem to be associated with a low grade chronic inflammation, possibly due to changes in the enzyme pathways of tryptophan metabolism mediated by IDO. It has been associated with disorders such as schizophrenia and depression14. Capuron et al 15 experimentally demonstrated that the level of infection, measured by the serum interleukin-6 and C-reactive protein levels, was related to a decrease in the concentration of tryptophan and an increase in the level of kynurenine in the blood, which suggested an increase in IDO-induced tryptophan catabolism. The increase in tryptophan metabolism is also associated with depression symptoms such as asthenia, lack of motivation, anorexia, and pessimism. Age correlates significantly with the concentrations of immunological markers and neuropsychiatric symptoms.
There are many studies on the importance of IDO in neoplastic disease, and we have contributed some experiences in our Research Unit16-18. It has been demonstrated that IDO plays a fundamental role in immune tolerance to neoplastic cells. Song et al19 demonstrated the inducing effect of kynurenine apoptosis on the human neoplastic cell line NK92 MI. In cultures, treatment with L-kynurenine induces growth inhibition due to the apoptosis, depending on the dose employed. And this opens the doors to new therapeutic targets, which are being explored20.
The role of IDO in inducing immune tolerance
IDO continues to be shown as an important molecule involved in immune tolerance, since it is one of the main actors involved in the inhibition of cell proliferation, including activated T cells, and thus playing a decisive role, that enables pregnancy, mediates in autoimmunity, and intervenes in neoplasms 20.
IDO was initially described as an essential enzyme for maternal-fetal tolerance. In 1998, Munn et al21 observed that gestation in mice was immediately rejected when an IDO inhibitor was administered to pregnant mice, and formulated the hypothesis that the expression of IDO was necessary to prevent immunological rejection of the fetus. They regarded IDO as a catabolizing enzyme of tryptophan, expressed in trophoblasts and macrophages. By means of tryptophan degradation, IDO could suppress the activity of T cells, preventing rejection of the fetus.
The control of the supply of micronutrients is a strategy to regulate the cell-mediated immune response. The cells that induce IDO, promote tryptophan metabolism, necessary for cell proliferation, and thus intervene in the immune response. In this sense, it has been shown that IDO activity promotes cell metabolism changes that affect the cellular and systemic response due to inflammatory or immunological stimuli in different clinical conditions such as neoplastic processes, chronic infections, autoimmune conditions, allergy syndromes, and transplants 22.
The anti-proliferative character of IDO in bacteria, protozoa, and tumor cells was described for the first time by Pfefferkorn in 198423 , and by Taylor and Feng in 199124.
IDO promotes the degradation of tryptophan into kynurenine within the cells. This has wide implications in the immune response of the body.
It is currently known that IDO forms part of a local, rapid immune regulation mechanism, called "metabolic immune regulation", by inducing a systemic immune tolerance, a protector of violent immune reactions 22.
The role of kynurenines in the immune system is evident, due to the immunosuppressive effect of IDO. There is evidence of an interaction between the kynurenine pathway, the cytokines , and the nervous system. IDO plays a key role in connecting the immune system with the kynurenine pathway. Pro-inflammatory stimuli activate the tryptophan metabolic pathway, while IDO, on promoting the degradation of tryptophan, exercises an immunosuppressive effect that includes inhibition of T cell functions, the activation of T cell regulators, and the inhibition of NK lymphocytes. There is a close relationship between the cytokines (IFN-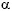 , IFN-
, IFN-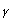 , TNF-
, TNF-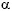 , TGF-
, TGF-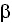 , IL-4 e IL-23) and the kynurenine system. IDO exercises a regulatory effect against the activation of antigen presenting cells mediated by interferon, with a feedback mechanism that modulates the immune response, maintaining homeostasis 25.
, IL-4 e IL-23) and the kynurenine system. IDO exercises a regulatory effect against the activation of antigen presenting cells mediated by interferon, with a feedback mechanism that modulates the immune response, maintaining homeostasis 25.
Oxidative stress and immunomodulation
IDO induces the production of reactive oxygen species (ROS) and nitric oxide (NO) radicals. Gostner et al26 noted that reactive oxygen and nitrogen species (ROS-RNS) and other active redox molecules fulfil key functions in immunity. Along with other pathogenic agent defense strategies, the redox reactions activate and modulate the immune response, and play an active role in the start and termination of cell repair processes.
Several pathways involved in the regulation of the immune response are regulated by redox mechanisms. The enzyme, nitric oxide synthase (NOS) induces the generation of nitric oxide (NO) in several cell types. This compound, despite its low reactivity, is a powerful antioxidant and inhibits the expression and function of IDO 27. The induction of IDO and NOS in the inflammatory response mediated by IFN-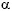 appears to be mutually regulated [28]. The absence of NO enables IDO to be more active in the inflammation site.
appears to be mutually regulated [28]. The absence of NO enables IDO to be more active in the inflammation site.
The regulation mechanisms due to redox activation ensure the correct development of the immune processes, and the imbalance in redox homeostasis, as may occur in chronic states of anoxia, leads to failures in the control mechanisms that favor the development of different pathological conditions, as we have observed in recent clinical studies29.
Interferon-gamma is the most powerful inducer of the ROS-RNS formation in target cells, such as macrophages. The immunomodulation that prompts tryptophan degradation through IDO, is initiated during the cellular immune response, concomitant to the production of ROS-RNS by immunocompetent cells. Treatment with the antioxidant N-acetyl-cysteine (NAC) completely protects the NK lymphocytes against the apoptosis induced by L-kynurenine. Furthermore, Song et al. 19 found that treatment with the inhibitor, z-VAD-fmk (pan-caspase z-Val-Ala-Asp (OMe) fluoromethylketone) and ZB4 (a Fas-antibody antagonist), slightly inhibits the apoptosis induced by L-kynurenine, which suggests that this induced apoptosis is mainly produced by a pathway mediated by ROS. Thus, the kynurenine resulting from the IDO activity may cause cell death through a ROS route in NK lymphocytes, by interaction processes between lymphocytes and cancer cells in the modulation of the immune response.
The complex interaction between tryptophan, IDO and the kynurenines themselves needs to be investigated further and in different pathological conditions. The capacity of the kynurenine pathway in the design of new treatment strategies is already being explored.
ACKNOWLEDGEMENTS
To Regional Health Service (Junta de Castilla y León), by grant GRS 389A09 and the Carlos III Health Institut (Ministerio de Sanidad) by grant PI10/00334
REFERENCES
1.- Ruddick JP, Evans AK, Nutt DJ, Lightman SL, Rook GA, Lowry CA. Tryptophan metabolism in the central nervous system: medical implications. Expert Rev Mol Med. 2006;8:1-27
2.- Salter M, Pogson CI. The role of tryptophan 2,3-dioxygenase in the hormonal control of tryptophan metabolism in isolated rat liver cells. Effects of glucocorticoids and experimental diabetes. Biochem J. 1985;229:499-504.
3.- Chen Y, Guillemin GJ. Kynurenine pathway metabolites in humans: disease and healthy States. Int J Tryptophan Res. 2009;2:1-19.
4.- Takikawa O. Biochemical and medical aspects of the indoleamine 2,3-dioxygenase-initiated L-tryptophan metabolism. Biochem Biophys Res Commun. 2005;338:12-19.
5.- Ball HJ, Sanchez-Perez A, Weiser S, et al. Characterization of an indoleamine 2,3-dioxygenase-like protein found in humans and mice. Gene. 2007;396:203-213.
6.- Metz R, Duhadaway JB, Kamasani U, Laury-kleintop L, Muller AJ, Prendergast GC. Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory compound D-1-methyl-tryptophan. Cancer Res. 2007;67:7082-7087.
7.- Werner-Felmayer G, Werner ER, Fuchs D, Hausen A, Reibnegger G, Wachter H. Characteristics of interferon induced tryptophan metabolism in human cells in vitro. Biochim Biophys Acta.1989;1012:140-147.
8.- Werner ER, Bitterlich G, Fuchs D, Hausen A, Reibnegger G, Szabo G, et al. Human macrophages degrade tryptophan upon induction by interferon-gamma. Life Sci. 1987; 41:273-280.
9.- Carlin JM, Borden EC, Sondel PM, Byrne GI. Interferon-induced indoleamine 2,3-dioxygenase activity in human mononuclear phagocytes. J Leukoc Biol.1989; 45:29-34.
10.- Brown RR, Ozaki Y, Datta SP, Borden EC, Sondel PM, Malone DG. Implications of interferon-induced tryptophan catabolism in cancer, auto-immune diseases and AIDS. Adv Exp Med Biol.1991;94:425-35
11.- Cozzi A, Zignego AL, Carpendo R, Biagiotti T, Aldinucci A, Monti M, Giannini C, Rosselli M, Laffi G, Moroni F. Low serum tryptophan levels, reduced macrophage IDO activity and high frequency of psychopathology in HCV patients. J Viral Hepat. 2006;13(6):402-408.
12.- van der Sluijs KF, Nijhuis M, Levels JH, Florquin S, Mellor AL, Jansen HM, van der Poll T, Lutter R. Influenza-induced expression of indoleamine 2,3-dioxygenase enhances interleukin-10 production and bacterial outgrowth during secondary pneumococcal pneumonia. J Infect Dis. 2006;193(2):214-222.
13.- Koenig P, Nagl C, Neurauter G, Schennach H, Brandacher G, Fuchs D. Enhanced degradation of tryptophan in patients on hemodialysis. Clin Nephrol. 2010;74(6):465-470.
14.- Müller N, Schwarz MJ. COX-2 inhibition in schizophrenia and major depression. Curr Pharm Des. 2008;14(14):1452-1465.
15.- Capuron L, Schroecksnadel S, Féart C, Aubert A, Higueret D, Barberger-Gateau P, Layé S, Fuchs D. Chronic low-grade inflammation in elderly persons is associated with altered tryptophan and tyrosine metabolism: role in neuropsychiatric symptoms. Biol Psychiatry. 2011;70(2):175-182.
16.- López AS, Alegre E, Díaz-Lagares A, García-Girón C, Coma MJ, González A. Effect of 3-hydroxyanthranilic acid in the immunosuppressive molecules indoleamine dioxygenase and HLA-G in macrophages. Immunol Lett. 2008;117(1):91-95.
17.- Cavia-Saiz M, Muñiz P, De Santiago R, Herreros-Villanueva M, Garcia-Giron C, Lopez AS, Coma-Del Corral MJ. Changes in the levels of thioredoxin and indoleamine-2,3-dioxygenase activity in plasma of patients with colorectal cancer treated with chemotherapy. Biochem Cell Biol. 2012;90(2):173-178
18.- Palamares T, De Lecea M, Armesto D, Cavia M, Al Kassam D, Alonso-Varona A. Relevance of indoleamine 2, 3-dioxygenase as prognostic biomarker in melanoma patients. In Eur J Cancer. 2013; 49: S863-S863.
19.- Song H, Park H, Kim YS, Kim KD, Lee HK, Cho DH, Yang JW, Hur DY. L-kynurenine-induced apoptosis in human NK cells is mediated by reactive oxygen species. Int Immunopharmacol. 2011;11(8):932-938.
20.- Curti A, Trabanelli S, Salvestrini V, Baccarani M, Lemoli RM. The role of indoleamine 2,3-dioxygenase in the induction of immune tolerance: focus on hematology. Blood 2009; 113:2394-2401.
21.- Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281(5380):1191-1193.
22.- Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013;34(3):137-143.
23.- Pfefferkorn ER. Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc Natl Acad Sci U S A. 1984; 81:908-912.
24.- Taylor MW, Feng GS. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991; 5: 2516-2522.
25.- Mándi Y, Vécsei L. The kynurenine system and immunoregulation. J Neural Transm. 2012;119(2):197-209.
26.- Gostner JM1, Becker K, Fuchs D, Sucher R. Redox regulation of the immune response. Redox Rep. 2013;18(3):88-94.
27.- Thomas SR, Terentis AC, Cai H, Takikawa O, Levina A, Lay PA, Freewan M, Stocker R. Post-translational regulation of human indoleamine 2,3-dioxygenase activity by nitric oxide. J Biol Chem. 2007;282:23778-23787.
28.- Alberati-Giani D, Malherbe P, Ricciardi-Castagnoli P, Köhler C, Denis-Donini S, Cesura AM. Differential regulation of indoleamine 2,3-dioxygenase expression by nitric oxide and inflammatory mediators in IFN-gamma-activated murine macrophages and microglial cells. J Immunol. 1997;159:419-442.
29.- Coma-del-Corral MJ, Muniz P, Terán-Santos J. Indoleamine-2,3-dioxygenase activity in plasma of patients with sleep apnea. Unpublished observations, 2013.
CORRESPONDENCIA:
Dra María Jesús Coma
Unidad de Investigacion
Hospital UNiversitario de Burgos
Avda. de las Islas Baleares 3
09006 Burgos.
Spain
Mail: mjcoma @ hubu.es
