Indice del volumen Volume index
Comité Editorial Editorial Board
Comité Científico Scientific Committee
THE PLACENTA IN A CASE OF LATE STILLBIRTH A MICROSCOPIC STUDY.
Olivar Clemente Castejón Sandoval, Anyana A González C,
Jaesmil J González S
Center for Research and Analysis Assistancel Teaching of the Nucleus Aragua (CIADANA)
Laboratory of Electron Microscopy.
Faculty of Health Sciences. University of Carabobo.
Aragua State. Maracay, Venezuela.
olivar.ciadanauc @ gmail.com
Rev Electron Biomed / Electron J Biomed 2015;1:45-52
RESUMEN
Objetivo: Interpretar hallazgos microscópicos en las vellosidades placentarias de un nacido muerto fetal tardío.
Material y Métodos: Placenta obtenida de parto pretérmino asociada a muerte fetal de causa desconocida a las 34 semanas de gestación. El peso placentario fue de 630 gr. Un protocolo de características se aplicó a placenta estudio y placenta control conteniendo: madurez vellosa, depósito de fibrinoide, edema, fibrosis estromal, calcificación, nódulos sincitiales, trombosis intervellosa, hiperplasia de la media muscular, infartos y vasos congestionados. Cinco biopsias por placenta fueron tomadas y de cada una cinco láminas fueron teñidas con Hematoxilina-Eosina. 20 campos por lámina fueron analizadas en un microscopio Standard Zeiss a 10X y 40X.
Resultados: Se observó infiltración de células mononucleares en la decidua pero no, en las vellosidades. La placenta estudiada demostró baja ramificación de las vellosidades con notoria inmadurez en sus abundantes vellosidades intermedias inmaduras. Edema, calcificación, invasión de células endoteliales hacia la luz de los vasos en vellosidades troncales, citotrofoblasto agrandado y fibrosis fueron hallazgos complementarios.
Conclusión: Varios cambios degenerativos que afectaron la maduración vellosa placentaria, como las bajas ramificaciones, interactuando con edema, infección y una posible merma del flujo sanguíneo útero-placentario pudieron constituir acontecimientos susceptibles de contribuir a la muerte fetal aquí reportada y analizada.
PALABRAS CLAVE: Vellosidad. Placenta. Microscopía. Mortinato fetal tardío.
SUMMARY:
Aim: To interpret microscopic findings in the placental villi of a late fetal stillbirth
Material and Methods: A placenta associated to idiopathic fetal death at 34 weeks' gestation, weighing 630 g and obtained from a cesarean preterm delivery was considered for microscopic analysis. Five specimens of placental villi were obtained from the studied placenta, subsequently processed for Haematoxylin - Eosin staining and finally observed in a Zeiss microscope with 10X and 40X objectives (20 fields/slide). The following features were considered for microscopic analysis: villous maturity, fibrinoid deposits, villous edema, stromal fibrosis, calcification, syncytial nodules, intervillous thrombosis, infarction, hyperplasia of the muscular media and congested vessels
Results: Infiltration of mononuclear cells was observed in decidua but not in placental villi. Studied placenta showed a low ramification of villi. Abundant immature intermediate villi revealed a noticeable immaturity. Edema, calcification, invasion of endothelial cells to lumen of vessels in stem villi, prominent cytotrophoblast and fibrosis were also found.
Conclusion: Severe degenerative changes affecting the maturity of placental villi, as low ramifications, interacting with edema, infection and a possible decrease in utero-placental blood flood could constitute events contributing to the fetal death here reported and analyzed.
KEY WORDS: Villi. Placenta. Microscopy. Late Stillbirth.
INTRODUCTION
Late fetal deaths -a fetus delivered with no signs of life after her mother has completed 24 weeks of pregnancy is here defined as stillbirth whilst intrauterine fetal death is particularly referred to fetuses with no signs of life in utero-, the largest group of perinatal mortality, have been associated with chronic or acute placental dysfunction. While the physiopathology of this dysfunction remains uncertain1, the role of the placenta in fetal death has become increasingly relevant in accordance with studies suggesting placental pathology as one of the main causes of fetal death2.
A triple risk model including the interplay of maternal, fetal and placental and stressor (venocaval compression from maternal supine sleep position) factors has been recently proposed for unexplained late stillbirth3.
Disorders of the placenta are associated with over 50% of stillbirth and they are frequently cited as the primary cause of death4.
Taking into account that the cause of death could be explained by placental examination alone, without fetal autopsy in 48% of the cases5, the assessment of the placenta becomes a relevant aid for stillbirth classification and its histological analysis, a key procedure in every case of stillbirth6. Histopathological placental lesions as inflammation, edema, vascular and degenerative lesions provide clues related with the cause of fetal death7.
The abovementioned features underscore the importance of examining the placenta, a fact sorely underestimated by obstetricians and general pathologists5.
In this context, this paper interprets morphological findings in the placental villi of a late stillbirth.
MATERIAL AND METHODS
A placenta associated to idiopathic fetal death at 34 weeks' gestation and obtained from a cesarean preterm delivery was considered for microscopic analysis. The time interval between fetal death and delivery was unknown.
The mother (aged 40 years) was previously and strictly informed following basic ethical principles of the Declaration of Helsinki as well as the rules of the Ethical Committee of our institution.
No signs of any illness were revealed during pregnancy.
Placenta weighed 630 g after draining its blood during 30 minutes immediately post delivery.
For accomplishing a descriptive, retrospective and no experimental study with non probabilistic sampling, five specimens of placental villi were obtained from the studied placenta and from a control one, subsequently processed for Haematoxylin - Eosin staining and finally observed in a standard clinical Zeiss microscope with 10X and 40X objectives (20 fields/slide).
The following features were considered in each one of the two placentas: villous maturity, fibrinoid deposits, villous edema, stromal fibrosis, calcification, syncytial nodules, intervillous thrombosis, infarction, hyperplasia of the muscular media and congested vessels.
In a term placenta, immaturity is defined by the prevalence of large villi with abundance of stroma and relatively small fetal vessels located centrally rather than on the periphery. Villous edema has a hydropic appearance with empty spaces below the syncitiotrophoblast plasma membrane. The fibrinoid deposits reveal the presence of fibrin plaques into a still unknown heterogeneous material containing trophoblast cells8-10.
RESULTS
An infiltrate of mononuclear cells in decidua could not be seen in placental villi.
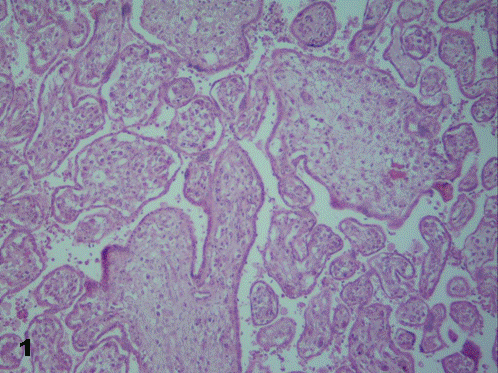
While in presence of a term placenta, strikingly numerous and barely ramified immature intermediate villi - corresponding more to earlier stages of gestation - were observed (Fig.1). Zones of infarction and calcification, fibrinoid necrosis and retroplacental hematomas were visualized. No chorangiosis was noted. A prominent and well-developed cytotrophoblast was present.
Placental villi were not surrounded by intervillous thrombosis. Some of them revealed vessels in the stromal region decreased in quantity. In immature intermediate villi a thinner trophoblast with interruptions and poor ramification was noted (Fig.2). In these villi impaired endothelial and muscular layer of vessels, and vessels tending to be closed or just disappearing, were noted (Fig. 3).
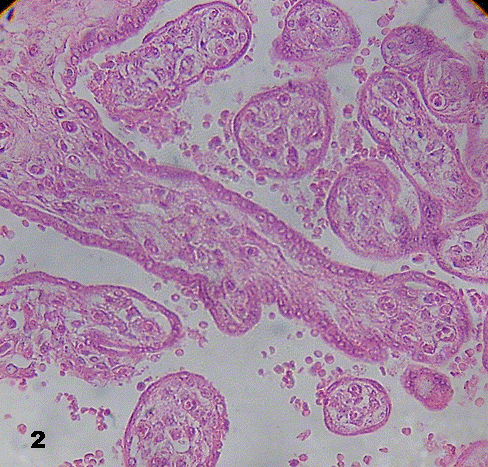
Fig.2. A region of immature intermediate villi with poor branching is seen across the picture.
A diminution of vessels may be observed. 400X.
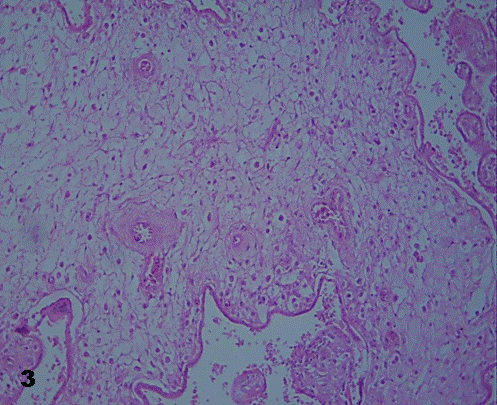
Fig.3.Stromal region of immature intermediate villi with subtrophoblastic edema
and vessels with perceptible degenerative changes. 640X.
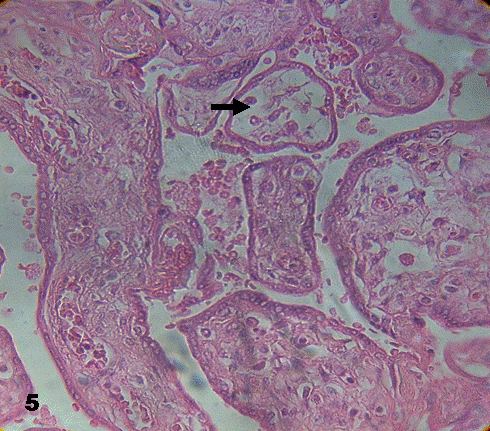
Stem villi put into evidence closed vessels. Some terminal villi contained stroma detached from the syncytium. There were zones with edematous immature intermediate apt to be seen in the subtrophoblastic region. The edema may also disorganize the stromal region and degenerated vessels were observed. Stem villi were occasionally observed with fibrotic regions and endothelial cells invading the vascular lumen (Fig.4). A notorious disorganization of stromal region was seen in some placental villi (Fig.5).Sometimes the placental villi appeared empty or without stroma.

Fig.4. A fibrotic region of stem placental villi with vessel (arrow)
whose cells of the endothelial layer have invaded the lumen of the vessel. 400X.
Numerous fibrotic placental villi were found. Immature intermediate villi contained remains of vessel structures in the stromal region. When regions of placental villi were visualized intact those vessels presented increased circulating nucleated red blood cells (NRBC).
To sum up, morphology of placental villi was usually as that observed in the upper left corner of Fig. 1, all of them edematous.
DISCUSSION
In the placenta of a 34 weeks' gestation stillbirth, low ramifications in numerous immature intermediate villi, degenerative vessel modifications, edema and stromal fibrosis may be pointing out low placental maturity with insufficiency of terminal villi8-9.
Placental villi with collapsed or diminished vessels with degenerative changes and most of them disappearing generate fibrotic villi and compromise fetal live by diminishing the interchange of gases or nutrients. Stromal fibrosis and calcifications are indicative that the fetus could be dead in utero for more than 7 days11. Changes in the syncytium and the prominent cytotrophoblast are similar to that observed in placental tissue cultures and those obtained after intrauterine fetal death. It has been suggested that the cessation of the fetal circulation causes these changes12. Placental histologic examination seems to be useful for determining the approximate time of death in many stillborn fetuses. If we found, by example, total luminal obliteration of vascular lumen of stem villi, in extensive form, the fetus has two or more weeks of approximate time of death13. In this case only some vessels were closed in stem villi.
Defect of maturation in placental villi or severely reduced vascularization and lack of syncytiocapillary membrane mostly after 35 weeks gestation can be a cause of fetal hypoxia and fetal death14. The placental vascular component is implicated in early and late intrauterine fetal death15. Infection is a common cause of stillbirth and chronic villitis is produced by infectious agents that inflame the placenta16. The nature of the infection here reported is unknown by us. Women older than 40 years of age have an increased risk for stillbirth being important risk factors obesity and poor antenatal care17. Increased NRBC detectable within fetal capillaries serve as a biomarker for significant fetal distress, hypoxia and ischemia18. These cells should not be seen at 34 weeks of gestation as it occurred here because of a probably response to placental hypoxia.
In this case defective placental maturation was observed with delayed maturation of the terminal villi or persisting villous immaturity. There is absent formation of terminal villi and syncytio-vascular membranes which leads to fetal hypoxia with increased risk of intrauterine fetal death and risk of recurrence stillbirth19. The edema affected noticeably the structure of the placental villi disorganizing the stromal region, localizing under trophoblastic zone and detaching the stroma by a ring-shaped zone of edema. Placental villous edema is the cause more frequent of fetal death before 28 weeks of gestation which has been explained according to factors placental, fetal and maternal20.
Closed vessels in stem villi could be provoked by edema. This stem villous edema has been seen associated with hypercoiled umbilical cord and stem obliterative endarteritis21. Emptied placental villi or without stromal region are product of edematous activity which destroy the internal organization of the placental villi. Stillbirth remains an enigma, in part due to lack of investigation but also due a failure to accurately identify its more than 50 causes of death22. In sum, the true cause of stillbirth and the mechanism leading to it23. These placental villi were not well adapted for the interchange of gases and nutrients. In this case is possible that fetal infection occurred but failed to trigger a fetal inflammatory response and the onset of labor. We do not know if these changes were produced before or after fetal death in the intrauterine environment24-25.
However, it is possible that events as infiltration of mononuclear cells, edema, calcification, invasion of endothelial cells, prominent cytotrophoblast and fibrosis occurred after fetal death11.
To conclude, severe degenerative changes affecting the maturity of placental villi, as low ramifications, interacting with edema, infection and a possible decrease in utero-placental blood flood could constitute events contributing to the fetal death here reported and analyzed.
REFERENCES
-
1. Paciencia M, Dolley P, Jeanne-Pasquier C, Jacob B, Sadfi A, Leseigneur P et al. Acute - placental dysfunction by villous - maturation defect and late-fetal mortality. J Ginecol Obstet Biol Reprod (Paris). 2008; 37:602-607.
2. Roescher AM, Timmer A, Erwich JJHM, Bos AF. Placental Pathology, Perinatal Death, Neonatal outcome, and Neurological Development: A systematic review. Plos One. 2014; 9: e 89419.
3. Warland J, Mitchell EA. A triple risk model for unexplained late stillbirth. BMC Pregnancy and childbirth. 2014; 14:142.
4. Gardosi J, Kady SM, McGeown P, Francis A, Tonks A. Classification of stillbirth by relevant condition at death (ReCODE): population based cohort study. British Med J. 2005; 331:1113-1117.
5. Tellefsen CH, Vogt C. How important is placental examination in cases of perinatal deaths? Pediatr Dev Pathol. 2011; 14:99-104.
6. Heazell AE, Martindale EA. Can post-mortem examination of the placenta help determine the cause of stillbirth? J Obstet Gynaecol. 2009; 29:225-228.
7. Larsen LG, Graem N. Morphological findings and value of placental examination at fetal and perinatal autopsy. APMIS. 1999; 107:337-345.
8. Benirschke K, Kaufmann P. Pathology of the human placenta. 4ta Ed. New York: Springer - Verlag, 2000.
9. Lewis SH, Perrin E. Pathology of the placenta. New York: Churchill Livingstone, 1999. 411p.
10. Kingdom J, Hupperts B, Seaward G, Kaufmann P. Development of the placental villous tree and its consequences for fetal growth. European J Obstet Ginecol and Reprod Biol. 2000; 92:35-43.
11. Fox H. Morphological changes in the human placenta following fetal death. J Obstet Gynaecol Br Common. 1968; 75:839-843.
12. Hustin J, Gaspard U. Comparison of histological changes seen in placental tissue cultures and in placental obtained after fetal death. BJOG. 1977; 84:210-215.
13. Genest DR. Estimating the time of death in stillborn fetuses: II. Histologic evaluation of the placenta; a study of 71 stillborns. Obstet & Gynecol. 1992; 80:585-592.
14. Stallmach T, Hebish G, Meter K, Dudenhausen J, Vogel M. Rescue by birth: defective placental maturation and late fetal mortality. Obstet & Ginecol. 2001; 97:505-509.
15. Bar J, Schreiber L, Ben-Haroush A, Ahmed H, Golan A, Kovo M. The placental vascular component in early and late intrauterine fetal death. Thromb Res. 2012; 130:901-905.
16. Syridou G, Spanakis A, Konstantinidou A et al. Detection of cytomegalovirus, parvovirus B19 and herpes simplex viruses in cases of intrauterine fetal death: association with pathological findings. J Med Virol. 2008; 80:1776-1782.
17. Mutz-Dehbalaie I, Scheier M, Jerabek-Klestil S et al. Perinatal mortality and advanced maternal age. Ginecol Obstet Invest. 2014; 77:50-57.
18. Redline RW. Elevated circulating fetal nucleated red blood cells and placental pathology in term infants who develop cerebral palsy. Hum Pathol. 2008; 39:1378-1384.
19. Treacy A, Higgins M, Kearney J M et al. Delayed villous maturation of the placenta: Quantitative assessment in different cohorts. Ped Develop Pathol. 2013; 16:63-66.
20. Castejón OC, Ali SK, Canache LZ. El Edema de la vellosidad placentaria en los casos de muerte fetal. Gac Med Caracas. 2006; 114:291-299.
21. Stanek J. Periarterial stem villous edema is associated with hypercoiled umbilical cord and stem obliterative endarteritis. Open J Obstet Gynecol. 2013; 3:9-14.
22. Macpherson TA (1990). Categorization of perinatal death-USA. Pediatr Pathol 10:v.
23. Mitchell EA, Heazell A. Proceedings of the stillbirth summit 2011 BMC Pregnancy Childbirth. 2012; 12:1-9.
24. Blackwell S, Romero R, Chaiworapongia T et al. Maternal and fetal inflammatory responses in unexplained fetal death. J Matern-Fetal Neonat Med. 2003; 14:151-157.
25. Ovalle A, Kakarieka WE, Correa PA, Vial PMA, Aspillaga MC. Estudio Anátomo-clínico de las causas de muerte fetal. Rev Chil Obstet Ginecol. 2005; 70:303-312.
ACKNOWLEDGMENTS
We are deeply grateful to the delivery room staff at the Maracay Central Hospital for their help in obtaining placenta, and to the administrative coordination of the Health Sciences Faculty of the Carabobo University of Nucleus Aragua Venezuela by the financial support for CIADANA.
CORRESPONDENCE:
Prof. Olivar C Castejón.
General Coordinator of the CIADANA. (Center for Research and Analysis Assistancel Teaching of the Nucleus Aragua) Laboratory of Electron Microscopy.
Faculty of Health Sciences. University of Carabobo
Aragua State.
Maracay, Venezuela.
Apdo. 4944.
olivar.ciadanauc @ gmail.com