Indice del volumen Volume index
Comité Editorial Editorial Board
Comité Científico Scientific Committee
Letters to the Editor / Cartas al Editor
THE VESSELS OF STEM VILLUS IN PLACENTA ASSOCIATED WITH OBESITY AND HYPERTENSION.
Olivar C. Castejón Sandoval
Center for Research and Analysis Assistancel Teaching of the Nucleus Aragua (CIADANA)
Laboratory of Electron Microscopy.
Faculty of Health Sciences. University of Carabobo.
Aragua State. Maracay. Venezuela.
olivar.ciadanauc @ gmail.com
Rev Electron Biomed / Electron J Biomed 2015;2:62-69
To the Editor:
During normal placental development stem villus originates from first-trimester mesenchymal villi, characterized by its loose stroma. Subsequently the vessels are centrally placed with fibrous connective tissue surrounding them1. When the placenta is structured these stem vessels arise from chorionic vessels. Stem villi has one to several large muscular vessels surrounded by a condensed fibrous adventitia containing superficially located paravascular capillaries, similar to vasa vasorum. The vessels of stem villi are composed of endothelium, muscular media and adventitia without elastic membrane2.
Obesity is a risk factor for hypertension. A corporal mass index > 29 increase that risk in 10%3. Hypertension is associated with vessel lesions (hyalinization or deposit of homogeneous, strongly eosinophilic and thinly granular material) and with proliferation of fibrous tissue or smooth muscle. These changes, described in uterine pathology4 but not in stem villi of placenta, lead to vessel wall thickening and lumen narrowing. Other physiopathologic mechanisms have been purposed for hypertension as low placental perfusion or ischemic-hypoxic processes producing lysis of endothelial cells, fragmentation of the endothelium and increase of the permeability. These events, originating endothelial dysfunction and occlusive compression of vessels, lead to low blood flow5. In this regard, an increase in the number and size of syncytial knots and a thinning of the syncytiotrophoblast and infarcts has been observed6.
The aim of this paper is to investigate degenerative changes occurring in stem villi vessels.
For carrying out this descriptive, retrospective and no experimental study with non probabilistic sampling, two placentas were obtained from two pregnant women at 38 weeks of gestation for microscopic analysis. Both pregnancies led to live-newborn with malformations. Women weighed 75 and 85 Kg and their hypertension exceed 90/150 mmHg. The placental weights were 600gr and 650gr after draining all their blood. Specimens were removed immediately post cesarean section from each other and fixed in buffered formaldehyde at 10% in the delivery room according to the conventional procedures for light microscopy.
Five specimens were obtained from each placenta and three slides per placenta were processed for Haematoxylin-Eosin staining. Twenty fields per slide were visualized in a standard clinical MC63A Zeiss microscope (Carl Zeiss, Oberkochen, West Germany) with 16X ocular and 10X - 40X objectives. The observations were focused in the stem villi and its results compared with samples removed from normal placentas.
A protocol linked to the structural characteristics of stem villi vessels was applied. Description of the normal stem villi here employed follows that of Benirschke and Kaufmann1.
Our result stated that stem villi showed vessels with degenerative changes in the endothelial layer. This appeared dilated or expanded in the stromal region and sometimes reveals aneurismal zones. Interruptions or perforations of this layer were seen affecting their continuity (Fig. 1).
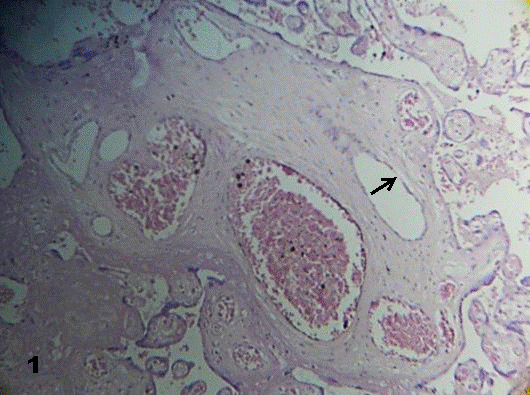
Fig.1. Stem villi vessels show aneurismal prolongations.
A thin and interrupted endothelial layer is seen (arrow). Some vessels are empty.160x H&E.
In other cases the endothelial layer has disappeared. The muscular media looked disorganized and its smooth muscle cells were orientated in different directions. These cells occupied the vessel lumen forming a net of cells (Fig. 2).
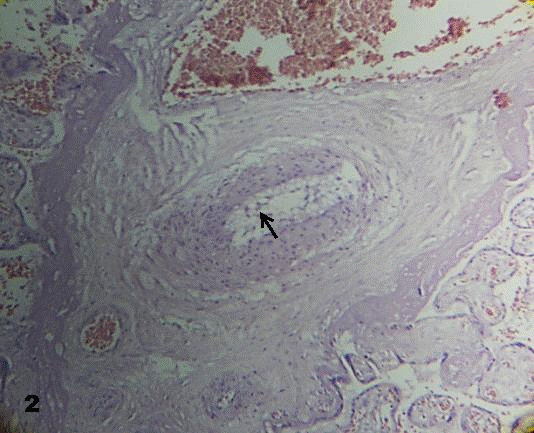
Fig.2. Endothelial layer is not observed.
Smooth muscle cells constitute a net in the lumen of a degenerative vessel (arrow). 160x H&E.
This lumen was obliterated by them in some of the stained sections. Images revealing early formation of thrombus were visualized (Figs. 3, 4).
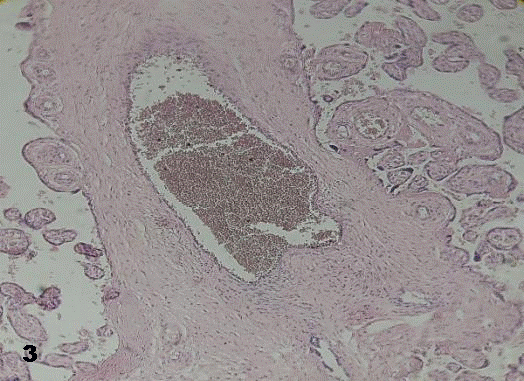
Fig.3. A growing thrombus into a stem villi vessel.100x H&E.
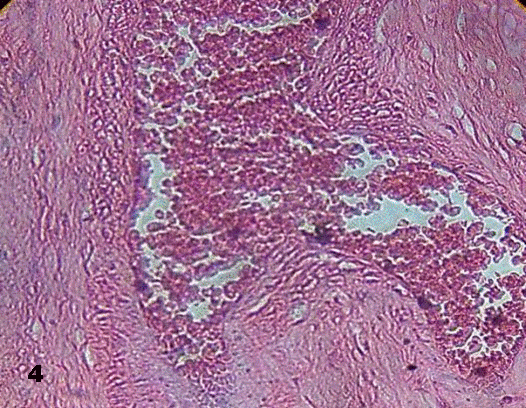
Fig.4.Changes in the wall of the vessel is observed
associated to stasis of erythrocytes in the lumen of vessel of a stem villi. 100x H&E.
Edema affected the muscular layer detaching the smooth muscle cells and almost all the stromal region of the stem villi. In the closed vessels smooth muscle cells replaced the endothelium. The vessel lumen was empty of erythrocytes in some of the stem villi and full of them in others. Edema disorganized the vessel adventitia (Fig. 5).
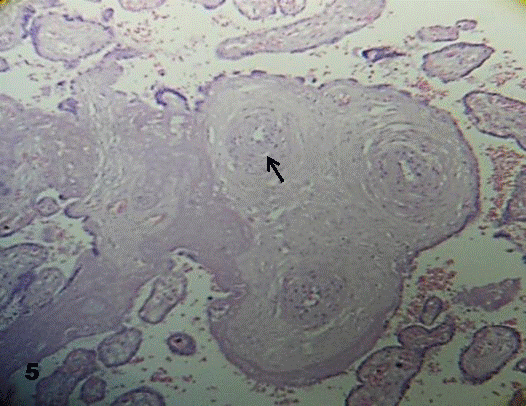
Fig.5. In this stem villi the edema affect the structure of stromal region
and the lumen of the vessel is narrow (arrow).160x H&E.
There were stem villi where vessels have disappeared and the stromal region appeared infiltrated by an accentuated deposit of fibrinoid (Fig.6).
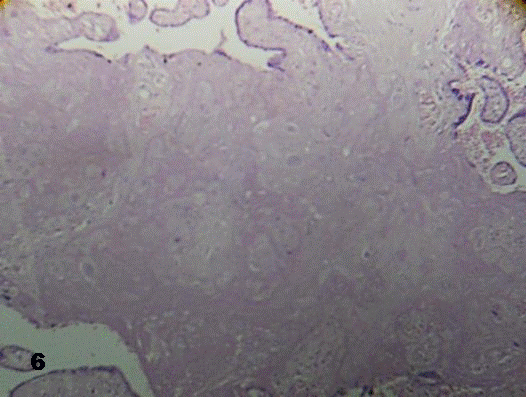
Fig.6. Stem villi infiltrated by deposition of fibrinoid associated with x-cells.
The vessels have disappeared.160x H&E.
Necrosis fibrinoid also was seen in placental villi connected with the stem villi. Peripheral smaller vessels in these stem villi, similar to vasa vasorum, revealed prominent and with same degenerative changes as those detailed above.
Aneurismal vessel dilatations were occasionally seen. Some of these stem villi revealed calcified in the stromal region supplied by vessels (Fig.7).
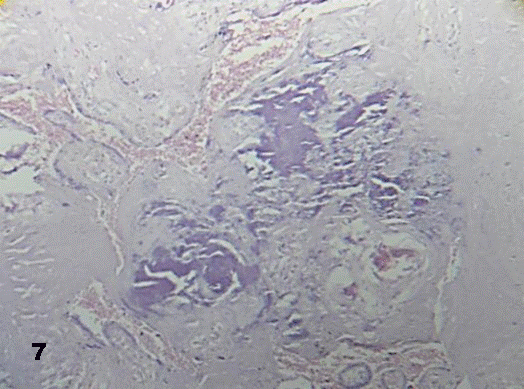
Fig.7. Stem villi with deposit of calcium and without vessels 160x H&E
Those vessels losing smooth muscle cells showed changes in lumen morphology revealing polygonal form.
Reduced utero-placental blood flow has been recognized in cases of severe hypertension6. In these cases, narrowing of fetal capillaries has been demonstrated7. This reduced blood flow provoke placental ischemia and low intervillous blood flow leading to degenerative functional changes as those observed in the endothelial layer due to bad placental perfusion. Abrupt diminution of the oxygen tension in this blood induce rapid and reversible vasoconstriction This could produce disorganization of the muscular media The duration of this vasoconstriction is inversely proportional to the oxygen tension and regulate the blood flow during life. Since hypoxia increases significantly the prostaglandins PGF2? during vasoconstriction this may explain the presence of closed vessels8.
Chronic hypoxia or alternate periods of hypoxia / reoxygenation within intervillous space is expected to trigger tissue oxidative stress and increase placental apoptosis9. Previous reports pointed out that an increased release of syncytiotrophoblastic micro particles of 0.2 to 2 um in size formed by plasma membrane blubbing during apoptosis are triggered in excess into maternal circulation10-11. These detached fragments, debris of trophoblast, micro villi from syncytium o syncytial microparticles could be considered as causing endothelial damage, among other factors12. Damaged endothelium leak out plasma to muscular media provoking disaggregation of smooth muscle cells and their disorganization associated with the edema originated. This also affected the adventitia of the vessel. These degenerative changes in the wall of the vessel lead to the formation of thrombosis. Occlusive vascular lesions as here observed can be seen in intrauterine growth retardation, intrauterine fetal demise, fetal abnormalities and thromboembolic disease2.
The observation of stem villi with some emptied vessels of erythrocytes indicate that fetus can not to absorb gases and nutrients or that placental villi are with bad perfusion promoting an ambient of hyperoxia which brake the process of branching angiogenesis resulting in filiform terminal villi1. Long standing hypertension with severe elevations can directly damage blood vessels. Changes in the intima and media can lead to significant narrowing of vessels and ischemia in placental villi which produce severe necrotic damage contributing with the disappearance of villi.
Perfusion of the placenta at abnormally low oxygen tension is associated with increased basal perfusion pressure, consistent with placental vasoconstriction13. Chronic vasoconstriction and increased intraluminal pressure could lead to vascular obliteration through progressive mural hyperplasia. Increased intraluminal pressure likely predispose to endothelial damage and luminal obliteration2. So, there are a prediction of that hypoxia stimulate the liberation from the placental villi to the maternal blood of a factor that interacts with the endothelium8.
Low blood flow in the intervillous space provoke a diminution of placental villi by fibrinoid necrosis of them or the formation of zones of infarct. This low blood flow can to be caused by deficient dilatation of utero-placental vessels associated with sharp arteriosclerosis during hypertension which would be increased in these cases associated with obesity.
Rupture of endothelial layer which is here seen dilated possibly contribute to a hemorrhagic endovasculitis. Blood vessel structure is altered in obesity with increase in vessel diameter14, limiting caliber and distensibility of vessel walls. Mechanisms that could affect placental blood vessel structure in maternal obesity. Enhanced understanding of normal and aberrant placental structure of vessel in early pregnancy of obese woman is required15.
On the other hand growing evidence in human and animal models of maternal obesity have indicated increased placental vascularity16, or chorangiosis which would be an adaptative response to low blood flow in the intervillous space.
Normal placental weights have increased over the last decades and this may correlate with increasing maternal obesity17. Increased placental weight and placental hypertrophy have been more common in obese groups18 and could be attributed to the increasing edema here observed.
Aneurysmal prolongation of vessel is a consequence of elevated blood pressure that modifies the wall of the vessel as seen in Fig.4.
Greater muscularity, higher numbers of neutrophils within the intervillous space and a heightened inflammatory response within the adipose and placental tissue has been demonstrated19. We found not this fetal environment of inflammation in our placentas associated with obesity and hypertension however recent studies suggest that heightened inflammatory response may be involved in adverse clinical outcomes during pregnancy20.
In obese individuals endothelial function is significantly impaired. Blood vessel structure is altered in obesity with an increase in vessel diameter, basement membrane thickness, vascular permeability, and vessel stiffness14. A progressive microvascular rarefaction develops21 increasing the risk of local tissue ischemia by atrophy and vessel diameter narrow. The mechanisms underlying impaired endothelial function in obese woman still are not well understood22.
In conclusion, degenerative changes in the wall vessel characterized by morphological changes in endothelial layer; edema, losing and invasion of cells in muscular layer as well as adventitia affected by edema have provoked a disorganization of the wall of the vessel with fibrinoid necrosis or calcification originating reduction of the blood flow which could to be affecting the normal development of the fetus.
REFERENCES
1. Benirschke K, Kaufmann P. Pathology of the human placenta. 4th edition. New York: Springer-Verlag; 2000.
2. Lewis SH, Perrin E. Pathology of the placenta. New York: Churchill Livingstone; 1999.
3. Reyna-Villasmil E, Prieto-Franchi M, Torres Montilla M, Reyna-Villasmil N, Mejías-Montilla J (2002). Alteración en el metabolismo de los carbohidratos y lípidos en mujeres que han sufrido preeclampsia. Rev. Obstet Ginecol Venez. 62:97-102.
4. Klopper A, Diczfalusy E. Foetus and Placenta. Oxford and Edinburgh: Blackwell Scientific Publications; 1969.
5. Zighelboim I, Guariglia D. Clínica Obstétrica. Segunda Edición. Caracas: Disinlimed C A; 2005.
6. Robinson NJ, Wareing M, Hudson NK, Blankley RT, Baker PN, Aplin JD et al (2008). Oxygen and the liberation of placental factors responsible for vascular compromise. Lab Invest. 88:293-305.
7. Salgado SS, Salgado MKR (2011). Structural changes in preeclamptic and eclamptic placentas - an ultrastructural study. J Coll Physicians Surg Pak. 21:482-486.
8. Redman CWG, Sargent IL, Starkey PM. La placenta humana. Guía para Perinatólogos. Barcelona: Masson SA, 1995.
9. Grill S, Rusterholz C, Zanetti - Dallenbach R, Tercanli S, Holzgreve W, Hanhn S et al (2009). Potential markers of preeclampsia - a review. Reprod Biol Endocrinol. 7:70-75.
10. Guller S (2009). Role of the syncytium in placenta-mediated complications of preeclampsia. Thromb Res. 124:389-392.
11. Redman CW, Sargent IL (2008). Circulating microparticles in normal pregnancy and pre-eclampsia. Placenta. 29A:S73-S77.
12. Goswami D, Tannetta DS, Magee LA, Fuchisawa A, Redman CWG, Sargent IT, et al (2006). Excess syncytiotrophoblast microparticle shedding is a feature of early - onset preeclampsia but not normotensive growth restriction. Placenta. 27:56-61.
13. Read MA, Boura ALA, Walters WAW (1995). Effects of variation in oxygen tension on responses of the human fetoplacental vasculature to vasoactive agents in vitro. Placenta. 16:667.
14. Zebekakis PE, Nawrot T, Thij S L, Balkestein EJ, van der Heijden-Spek J, Van Bortel LM et al (2005). Obesity is associated with increased arterial stiffness from adolescence until old age. J Hypertension. 23:1389-1846.
15. Higgins L, Greenwood SL, Wareing M, Sibley CP, Mills TA (2011). Obesity and the placenta: A consideration of nutrient exchange mechanisms in relation to aberrant fetal growth. Placenta. 32:1-7.
16. Farley D, Tejero ME, Commuzzie AG, Higgins PB, Cox L, Werner SL, et al (2009). Feto placental adaptations to maternal obesity in the baboon. Placenta. 30:752-760.
17. Swanson LD, Bewtra C (2008). Increase in normal placental weights related to increase in maternal body mass index. J Matern Fetal Neonatal Med. 21:111-113.
18. Wallace JM, Horgan GW, Bhattacharya S (2012). Placental weight and efficiency in relation to maternal body mass index and the risk of pregnancy complications in woman delivering singleton babies. Placenta. 8:611-618.
19. Roberts KA, Riley SC, Reynolds RM, Barr S, Evans M, Statham A, et al (2011). Placental structure and inflammation in pregnancies associated with obesity. Placenta. 32:247-254.
20. Denison FC, Roberts KA, Barr SM, Norman JE (2010). Obesity, pregnancy, inflammation, and vascular function. Reproduction. 140:373-385.
21. Stepp DW, Belin De Chantemele EJ (2007). Structural remodeling in limb circulation: impact of obesity and diabetes. Microcirculation. 97:2601-2610.
22. Walsh SW (2007). Obesity: a risk factor for preeclampsia. Trends Endocrinol Metab. 18:365-370.
ACKNOWLEDGMENTS
The author deeply acknowledges to the Medical staff of Gynaecology and Obstetrics of the Maracay Central Hospital and "Carabaño Tosta" Hospital of the IVSS; to the Administrative Coordination, Faculty of Health Sciences Nucleus Aragua by their financial support for CIADANA, and to TSU Laury R Gutierrez S by the transcription of the manuscript.
CORRESPONDENCE:
Prof. Olivar C Castejón.
General Coordinator of the CIADANA. (Center for Research and Analysis Assistancel Teaching of the Nucleus Aragua) Laboratory of Electron Microscopy.
Faculty of Health Sciences. University of Carabobo
Aragua State.
Maracay, Venezuela.
Apdo. 4944.
olivar.ciadanauc @ gmail.com
