Indice del volumen Volume index
Comité Editorial Editorial Board
Comité Científico Scientific Committee
IMMATURITY IN PLACENTAL VILLI AFFECTED BY CHIKUNGUNYA VIRUS.
Olivar C Castejón S.
Center for Research and Analysis Assistancel Teaching of the Nucleus Aragua (CIADANA). Laboratory of Electron Microscopy.
Faculty of Health Sciences. University of Carabobo.
Aragua State. Maracay. Venezuela
olivar.ciadanauc @ gmail.com
Rev Electron Biomed / Electron J Biomed 2015;3:21-29
RESUMEN: INMADUREZ EN LA VELLOSIDAD PLACENTARIA AFECTADAS POR VIRUS CHIKUNGUNYA
Objetivos: Un estudio sobre la vellosidad intermedia inmadura fue realizado en placenta de embarazada infectada por el virus Chikungunya usando las tecnicas de microscopia de luz con el proposito de obtener un mejor entendimiento de la inmadurez placentaria provocada por estos virus.
Metodos: Placenta fue obtenida a las 37 semanas de embarazo de una paciente de 32 años de edad infectada durante el tercer trimestre de embarazo con bajo incremento de peso.El diagnostico fue confirmado por un test de seroconversion(Elisa IgM/IgG) y deteccion de acidos nucleicos(RT-PCR).La serologia de la paciente fue negativa para otros micro-organismos,sin otras enfermedades o malformaciones siendo negativa a los seis meses del nacimiento. Cinco pequeños fragmentos fueron tomados de la superficie maternal de la placenta y tres laminas por especimen fueron preparadas segun tecnica de microscopia de luz.El mismo procedimiento fue empleado para una placenta normal como control y 30 laminas histologicas fueron teñidas con H&E para su observacion.
Resultados: Numerosas vellosidades intermedias inmaduras fueron observadas con deposicion de fibrinoide,hipervascularizadas,con vasos hemorragicos y degenerados,edema,ausencia de celulas de Hofbauer,zonas claras o regiones de lisis en la region estromal de la vellosidad,fibrosis o colagenosis,interrupciones del sincitio y calcificacion.
Conclusion: Un fuerte ataque viral afecta notablemente la vellosidad intermedia inmadura de la placenta provocando una disminuida formacion de vellosidades intermedias maduras con vellosidaes terminales lo cual perturbaria el normal intercambio de gases y nutrientes que mantienen el desarrollo fetal.
PALABRAS CLAVE: Inmadurez. Vellosidad placentaria. Virus Chicungunya
SUMMARY
Objectives: An study on the immature intermediate villi was realized in placenta of woman pregnancy infected by Chikungunya virus using stains of light microscopy with the purpose of to obtain a better understanding of the placental immaturity provoked by these viruses.
Methods: Placenta was obtained to the 37 weeks of pregnancy of patient of 32 years old infected during the third trimester of pregnancy with low increase of weight. The Chikv diagnostic was confirmed by seroconversion test (Elisa IgM/IgG) and nucleic acids detection (RT-PCR). The serology of this patient was negative for others microorganisms without other diseases or malformations being negative to the six weeks of birth. Five small fragments were taken of the maternal surface of the placenta and three slides by specimen were prepared for light microscopy. The same procedure was employed for normal placenta as control and 30 histological slides were stained with H&E for their observation.
Results: Numerous immature intermediate villi were observed with little focus of fibrinoid deposition, hipervascularized with hemorrhagic and degenerative vessels, edema, absence of Hofbauer cells, clear areas or regions of lysis in stromal region, fibrosis or collagenosis, interruptions of the syncytio and calcification.
Conclusion: a strong viral attack of CHikv affect noticeably the immature intermediate villi of placenta provoking a diminished formation of mature intermediate villi with terminal villi which perturbs the normal interchange of gases or nutrients that maintain the fetal development.
Key words: Immaturity Placental villi Chikv
INTRODUCTION
The characteristics of Chikungunya virus (CHIKV) or chikunguña as an Arbovirus transmitted by arthropods has been briefly described in placenta in a preliminary work1. In this new study persistence of immaturity was found which require attention notable. This case of villous baddevelopment is defined as persisting immaturity provoked by CHIKV in which the villous tree is composed mainly of immaturity intermediate villi with various degrees of admixture of mature intermediate villi.
Villous maturation is retarded in this condition2. Immature intermediate villi of large caliber persist in presence of CHIKV and the formation of new villi is decelerated. So, the immature intermediate villi are not producing mature intermediate villi which could to originate terminal villi where is realized the interchange of gases and nutrients1. CHIKV produces cytopathic effect in a variety of cellular lines as Vero cells, BHK-21 and HeLa3 and recently has been described their effect on placental villi1. The fever produced by this virus presents a mortality of 0,4% in child under age of a year4 and there are reports of spontaneous abortus after infection by CHIKV in the mother5. CHIKV affect the structure of the placental villi in the syncytio, stromal zone and blood vessels of the villi provoking severe degenerative changes.
This virus RNA of the family togaviridae and generus alfavirus produces febril disease with arthritis, pain of back and pain of head. Isolated by first time in Bangkok, Tailandia in 1958. 45 cases were reported in the year 20146 here in Venezuela, 2 autochthonous and 43 importated. This virus has circulated in the Americas since 2013 and no adaptive mutations have occurred7. Althought others studies indicate that mutations of this virus enhances viral dissemination and transmissibility8. Has been documented maternal transmission to the newborn when the mother presents fever days before or in the moment of the delivery. Caesarian no avoid the transmission. By this reason pregnancy womans with chicungunya are a group of risk9 and our proposal is to describe the changes provoked by CHIKV on the immature intermediate villi using light microscopy.
MATERIAL AND METHODS
Two groups of population of placental villi were taken of placenta normal and placenta study. The group study proceed from hospitalary institution whose placenta was obtained to the 37 weeks of pregnancy, of pacient of 32 years old, of low education level and economic resources who was infected during the third trimester of pregnancy, with an poor increase of weight of only 6 kg.
The newborn was born alive with 51 cm and 3600gr. The placenta normal was obtained at 38 weeks of pacient with an increase of weight of 10 kg, without antecedent of disease. The Chikv diagnostic was confirmed by seroconversion test (Elisa IgM/IgG) and nucleic acids detection (RT-PCR). The serology of pacient with placenta study was negative for Hepatitis B, C, cytomegalovirus, Epstein Barr virus, rubella and toxoplasmosis. Without other metabolic disease, genetic, parasitary, or with malformations and being seronegative to the six weeks of birth.
The infected woman pregnancy had knowledge of informed consent and approval by the ethical committee of the hospitalary institution for the realization of this investigation according to the Helsinki declaration. Diagnostic was made by Micro-Elisa of fourth generation, with equipment automatic AXSYM (Abbot, EUA) and confirmatory Western Blot Assay (Germany-Singapore Science Park). Of each placenta were taken five small specimens of the maternal surface selected to the random from the region central parabasal in the vertical plane. Three slides by specimen were prepared for light microscopic, 30 histological slides in total which were stained with H&E for their observation.
RESULTS
Numerous immature intermediate villi were observed in diverse grade of maturation near basal plate associate with little focus of fibrinoid deposition (Fig. 1).
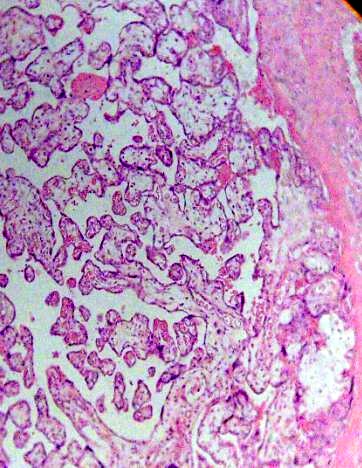
Fig. 1. Placental villi are seen with noticeable immaturity. H&E 100x.
These in some cases presented hipervascularized stroma with vessels that appear to have destroyed his layer and erythrocytes are dispersed to the stroma (Fig. 2).
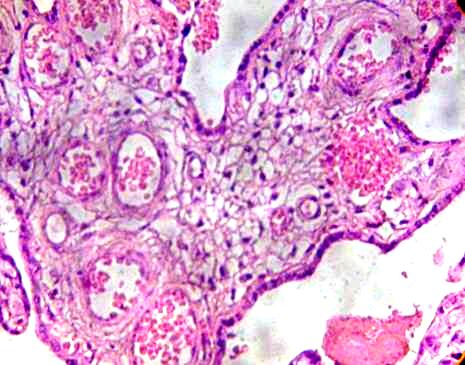
Fig. 2. Immature intermediate villi is observed with numerous vessels. H&E 400x.
This stroma can to be fibrosed in part and subthrophoblastic edema is noted (Figs. 3, 4).
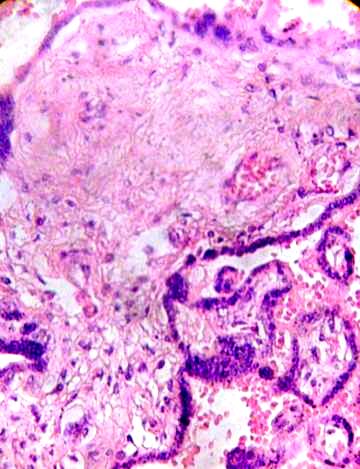
Fig. 3. Clear zones indicating lysis in the stromal region are observed.
Fibrotic region and damaged vessels can be seen. H&E 400x.
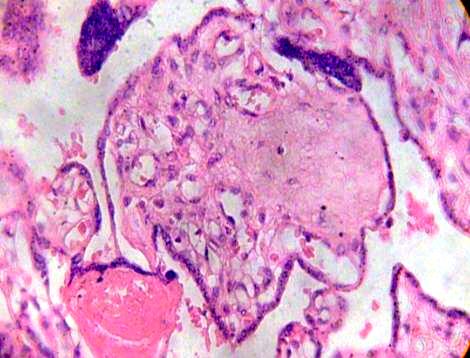
Fig. 4. Syncytial nodule is noted in fibrotic and edematous villi. H&E 400x.
Images so seen shows immature intermediate villi associated with fibrotic branchs (Fig. 5).
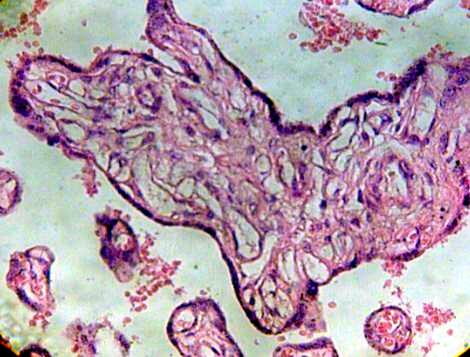
Fig.5 Immature intermediate villi without Hofbauer cells and blood vessels degenerated H&E 400x.
Immature intermediate villi were seen with fluid-filled channels without Hofbauer cells inside the channels. Deteriorated blood vessels are observed (Fig. 6).
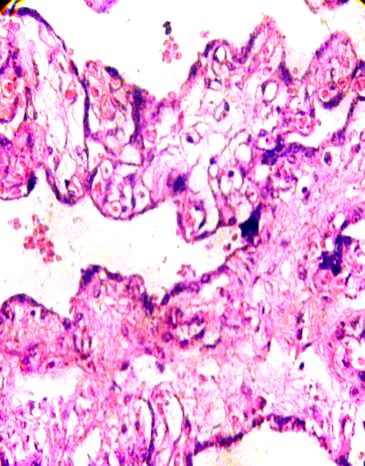
Fig.6. A fibrotic branch of placental villi is associated with another three
at the right which stay in fusion syncytial. H&E 400x.
Many of these contain degenerative syncytium which is noted interrumped and thinner (Fig. 7). Zones of calcification were observed surrounding to debris of immature intermediate villi (Fig. 8).
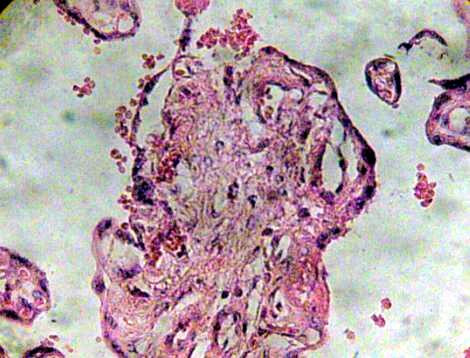
Fig. 7. Region of immature intermediate villi in degeneration. H&E 400x.
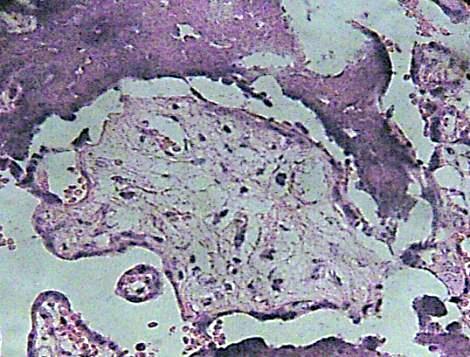
Fig. 8. Debris of immature intermediate villi can be seen associate with areas of calcification. H&E 400x.
DISCUSSION
The viral attack has transformed the placental tree in a structure diminished in ramifications villous persisting a large population of immature intermediate villi which maintain a diverse grade of immaturity1, 2. The hipervascularized stroma of these villi has originated a chorangiosis in response to a low efficiency of oxygen transfer to the fetal circulation in the villi10.
Fibrosis or collagenosis was also observed in skeletal muscle fibers and muscle satellite cells probably could be eliminated by a direct cytopathic effect as also could to have produced in the results here showed11, 12.
Lysis of the syncytial plasma membrane by the viruses on it could produce holes and the entrance of fluids in these since the intervillous space disorganize the stromal region1. Syncytial fusion is a consequence of the viral cytophatic effect.
The absence of Hofbauer cells into chanels in some immature intermediate villi is contrary to the observed persistence by long time of CHIKV into macrophages as cellular reservoirs during CHIKV infection in vivo potentially explaining long-lasting syntoms in humans11. In our work the attack viral ha eliminated in part many macrophages into channels. This indicates a higher viral attack to the placenta. Changes degenerative noted in the blood vessels of the stroma transform the placental villi in a fibrotic structure. Macrophages and endothelial cells are contributing significantly to the production of CHIKV progenie and are much more permissive to CHIKV infection that another cells13. Numerous interruptions of the syncytium have provoked the entrance of fluids to the stromal region contributing with the death of the villi. A simultaneous process of distrophic calcification are accompanying these events.
As has occurred in patients with chronic inflammatory rheumatism CHIKV infection has a destructive effect on the human placenta. The pathophysiology of this infection has been investigated recently. Vertical contamination maternal-fetal via breaches that arise at the term of pregnancy and during parturition which lead to maternal-fetal exchanges occurs in the placental barrier. But this trasplacental transmission is not observed in macaques and mice. This vertical transmission probably abortifacient is suspected to be directly linked to the fetal deaths5.
This study no revealed extensive infiltration with mononuclear cells as has been described by Labadie et al18 in lymphoid organs, liver, joint and muscle. Probably this is produced because this infection course with leucopenia, lymphopenia or thrombocytopenia. If well it has been suggested that macrophages are not a general target of infection19 we have found a diminished quantity of them in the channels of immature intermediate villi. Fibroblast also have been seen diminished since these cells are a target for CHIKV19.
Viral maternal-neonatal transmission is frecuently observed in viremic mothers around the term of pregnancy, when the highly viremic maternal blood (mean viral load 1.5 million copies / ml of plasma) can be in contact with placental barrier breaches resulting from uterine contractions during labor.
This transmission continues being a mystery since in one case of 19 transmitters gave birth to dizygous twins: one neonate remained uninfected, whereas the other became infected20. The mechanism of infection remains undefined. Couderc et al19 has showed that human syncytiotrophoblast is refractory to chikungunya infection, however we have found a syncytium in degeneration of immature intermediate villi. Since our case was in a women with lower educational level and low recourses it is possible that lack of basic knowledge about disease prevention might be influencing these results.
As also has been observed in acute arthritis necrosis and collagenosis or fibrosis are produced when CHIKV infection alters the metabolism of the connective tissue in the stromal region of placental villi21. Dystrophic calcification could to be observed also in skeletal muscle of newborn and 14 day old mice inoculated subcutaneously with CHIKV12 during persistence of the virus in this tissue for several days.
It has been reported that flavivirus infection results in significant loss of peroxisomes in mammalian cells which may indicate that targeting of peroxisomes is a key strategy used by virus to subvert early antiviral defenses. So, these viruses evade the innate immune system during early stages of infection22.
In conclusion, a strong viral attack by CHIKV has prevented the normal process of maturation in the placental tree provoking degenerative changes that could to damage the interchange of gases or nutrients affecting the normal development of the fetus.
REFERENCES
-
1. Castejón S OC. The placenta in a case of pregnant woman infected by chikungunya virus. J Virol Retrovirol 2016; 2:1-4.
2. Benirschke K, Kaufmann P. Pathology of the human placenta. 4ed. New York: Springer-Verlag, 2000.
3. PAHO/CDC. Preparedness and response for chikungunya virus introduction in the Americas. Washington, 2011; 159p.
4. Torres J, Gonzalez Y. (2014). Virus chikungunya. R Dominicana: Infección Neonatal. https://es.noticias.yahoo.com/dominicana-m-100nacidos con Chikungunya-164021896.html
5. Touret Y, Randrianaivo H, Michault A et al. Early maternal-fetal transmission of the chikungunya virus. Presse Med. 2006; 35:1656-1658.
6. Jimenez CME. Fiebre por virus Chicungunya. Dirección general de Epidemiologia. Mexico, Salud-Secretaria de Salud, 2014; 32p.
7. Kautz TF,Diaz GEE,Erasmus JH et al. Chicungunya virus as cause of febrile illness outbreak,Chiapas,Mexico,2014.Emerg Infect Dis 2015; 21:2070-2073.
8. Das B, Sahu A, Das M et al. Molecular investigations of Chicungunya virus during outbreaks in Orissa, Eastern India in 2010. Infect Genet Evol 2012; 12:1094-1101.
9. WHO, Center of Press (2014).Chicungunya.327.Descriptive note, Oct.
10. Sourisseau M, Schilte C, Casartelli N et al. Characterization of remerging Chikungunya virus. Plos Pathog 2007; 3:e89.
11. Ozden S, Huerre M, Pierre RJ et al. Human muscle satellite cells as targets of Chicungunya virus infection. Plos One 2007; 2:e527.
12. Ziegler SA, Lu L, Travassos Da Rosa APA et al. An Animal model for studing the pathogenesis of Chikungunya virus infection. Am J Trop Med Hyg 2008; 79:133-139.
13. Van Duijl-Richter MKS,Hoornweg TE, Rodenhuis ZIA et al. Early events in Chikungunya Virus infection-From virus cell binding to membrane fusion. Viruses 2015; 7:3647-3674.
14. Mattar S,Miranda J,Pinzon H et al. Outbreak of ChiKungunya virus in the north Caribbean area of Colombia: Clinical presentation and phylogenetic analysis. J Infect Develop Ctries 2015; 9:1126-1132.
15. Kumar A,Mamidi P,Das I et al. A novel 2006 indian outbreak strain of Chikungunya virus exhibits differents pattern of infection as compared to prototype strain. Plos One 9; 2014:e85714.
16. Gardner J, Rudd PA,Prow NA et al. Infections Chikungunya virus in the saliva of Mice, Monkey S and Humans. Plos One 2015; 10:e0139481.
17. Couderc T, Lecuit M. Chikungunya virus pathogenesis: from bedside to bench. Antiviral Research 2015; 121:120-131.
18. Labadie K,Larcher T,Joubert C et al. Chikungunya disease in nonhuman primates involves long-term viral persistence in macrophagues. J Clin Invest 2010; 120:894-906.
19. Couderc T, Chrétien F, Schilte C et al. A mouse model for Chkungunya:Young age and inefficient type-I Interferon signaling are risk factors for severe disease. Plos Pathog 2008; 4:e29.
20. Gérardin P, Barau G,Michault A et al. Multidisciplinary prospective study of mother-to-child Chikungunya virus infections on the island of La Reunion.Plos Med 2008; 5:e60.
21. Lokireddy S,Vemula S,Vadde R. Connective tissue metabolism in Chikungunya patients. Virol J 2008; 5:31.
22. You J, How S, MaliK-Sony N et al. Flavivirus infection impairs peroxisomes biogenesis and early antiviral signaling. J Virol 2015; 89:12349-12361.
CORRESPONDENCE:
Prof. Olivar C Castejón.
General Director of the CIADANA.
Faculty of Health Sciences.
University of Carabobo - Aragua State -
Maracay, Venezuela.
Apdo. 4944.
E-mail: olivar.ciadanauc @ gmail.com
