Indice del volumen Volume index
Comité Editorial Editorial Board
Comité Científico Scientific Committee
INFECTION BY CORONAVIRUS IN THE PLACENTAL VILLI
Olivar Clemente Castejon Sandoval PhD.1
Luzardo A Canache C, PhD2
Aquiles Lara A, PhD3
Jesus Veroes, PhD.4
1Director of the Center for Research and Analysis Assistancel Teaching of the Nucleus Aragua (CIADANA). Full professor in Biology, Msc. Laboratory of Electron Microscopy. Faculty of Health Sciences, University of Carabobo. Aragua State. Maracay.
2The Floresta Professional Center. The Floresta Maternity Annex. Maracay.
3Maracay Medical Center. Pathology Laboratory. Maracay
4Female Health Unity. Paula Saint Medical Group. Caracas -The Cafetal.
Venezuela.
Email: olivar. ciadanauc @ gmail. com
Rev Electron Biomed / Electron J Biomed 2019;3:42-49.
RESUMEN
El síndrome respiratorio agudo y severo provocado por el coronavirus 2(SARS-CoV2),un nuevo agente zoonótico brotando actualmente, ha sido estudiado por su efecto sobre la vellosidad placentaria en paciente de 29 años de edad quien tuvo Covid-19 a las 36 semanas de embarazo y cuya placenta fue descrita con microscopía de luz.
Deciduitis,villitis,desorganización de la región estromal de la vellosidad,citotrofoblastos hipertrofiados fusionados, calcificación distrófica, deposición de fibrinoide, necrosis celular, vellosidades destruidas,restos de vellosidad troncal fibrótica en el espacio intervelloso,numerosos nódulos sincitiales y extensas zonas de sincitio con numerosos núcleos fueron encontrados.
Este ataque viral contra el árbol velloso puede resultar en una aumentada morbilidad y mortalidad entre las mujeres embarazadas con el potencial para adversamente afectar al feto y neonato en desarrollo.
PALABRAS CLAVE: SARS-CoV2. Cambio placentario.
ABSTRACT:
The severe acute respiratory síndrome coronavirus 2 (SARS-CoV2) a newly emerging zoonotic agent, has been studied by their effect on the placental villi, in patient of 29 years old who had Covid-19 at 36 weeks of pregnancy whose placenta was described with light microscopy.
Deciduitis, villitis, disorganization of the stromal region of the villi, fusioned cytotrophoblasts hypertrophied, distrophic calcification, fibrinoid deposition, cellular necrosis, destroyed villi, fibrotic rest of stem villi in the intervillous space, numerous syncytial knots and extensive zones of syncytium with numerous nuclei were found.
This viral attack against the villous tree can result in increased morbidity and mortality among pregnant women with the potential to adversely affect the developing fetus and neonate.
KEY WORDS: SARS-CoV2. Change Placental
INTRODUCTION:
The coronaviruses are virus pleomorphic or spherical of 80-220nm, with an icosahedral core structure within which is a helical nucleocapsid. Their genome consists of a single molecule of linear positive sense, single-stranded RNA, 23-31 kb in size and is very infectious. Replicate in the cytoplasm and are released by exocytosis1.
It is known that viruses circulating in the maternal bloodstream enter the placenta from uterine arteries, circulate in the intervillous space, and can pass to the fetus through the chorionic villi tree where they eventually enter the fetal circulation. It appears that the absence thus far of maternal-fetal transmission of the SARS-CoV2 virus during the covid-19 pandemic is similar to other coronaviruses, and is also consistent with the extreme rarity of suggested or confirmed cases of intrauterine transmission of other respiratory RNA viruses. If intrauterine transmission of SARS-CoV2 eventually occurs, it will be a rare event2.
There is evidence of SARS-CoV2 vertical transmission when the infection ocurrs in the third trimester of pregnancy and an increased risk for premature delivery. It is of 3. 2 % (23/936) by infant nasopharyngeal swab testing. Although it has been a point of a recent debate concluding that there is no evidence of vertical transmission and no known cases have been noted in similar coronavirus as SARS and MERS.
The coexpresion of ACE2 and transmembrane serine protease2 (TMPRSS2), receptors of coronavirus in the placenta, are in a minimal number of placental cells. The chorioamniotic membrane of the third trimester exhibits minimal coexpresion of both proteins. Nonetheless, others entry mediators could to be active3.
The RT-PCR which was positive in the amniotic fluid and throat swab at 24 hours, for the baby born at 32 weeks gestation to the mother with symptomatic Covid-19 strongly raises the possibility of vertical transmission4.
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) formerly knowed as 2019 novel coronavirus (2019-nCoV) is a newly emerging zoonotic agent that appeared in December 2019 and causes the coronavirus disease 2019 (COVID-19)5.
This emerging disease course with fever, cough, dyspnea, shock, lymphopenia, high erythrocyte sedimentation rate and can to be fatal 6.
The preliminary findings in covid-19 positive mothers describe fetal vascular malperfusion provoqued by intervillous thrombus, villous stromal-vascular karyorrhexis, avascular villi, intramural fibrin deposition in stem vessel, decidual vasculopathy, chronic villitis and focal chorangiosis7.
Numerous placental lesions have been associated with SARS-CoV2 infection during third trimester of pregnancy as fetal vascular malperfusion, maternal vascular malperfusion, perivillous fibrin deposition, increased syncytial nodules, chorioamnionitis, chronic villitis, intervillositis, chorangiosis, delayed villous maturation, deciduitis, villous oedema, placental abruption, and infarction8.
SARS-CoV2 induce syncytial cell formation and cell tight junction destruction, extensive cell death caused by apoptosis or necrosis, formation of numerous pleomorphic double-membrane vesicles in the cytoplasm of infected cells and aggregation of organels close to the apical surface9.
Pathologic studies on biopsy samples of lung, liver, heart, obtained of death Covid-19 pacients have revealed that the lung is the main affected tissue with pathological changes as hyperplasia of type II pneumocytes, damage to the alveolar epithelial cells, formation of hyaline membrane and diffuse alveolar damage10.
Thrombotic microangiopathy, accumulations of CD4 mononuclear cells around small thrombotic vessels and notable hemorrhage as cause of death in these pacients, presence of megacariocytes in the lung, platelet aggregation, fibrin deposition and clot formation have been found11.
Others morphological changes observed in the placenta are:accelerated villous maturation, distal villous immaturity, vasculitis, intervillous hematoma and subchorial hematoma12. Fibro-myxoide exudates, mononuclear inflammatory infiltrates dominated by lymphocytes, large nuclei, prominent nucleoli. No obvious intranuclear or intracitoplasmic viral inclusions, over activation of T cells and limphopenia13.
In the placentas of two women who were convalescing from SARS-CoV2 infection in the third trimester of pregnancy, these were highly abnormal with extensive fetal thrombotic vasculopathy, with areas of avascular chorionic villi as chronic findings of fetal vascular malperfusion. These pregnancies were complicated with IUGR14.
Howeber, a characteristic placental pathology has not been clearly demonstrated in placentas exposed to SARS-CoV2 since ACE2 viral receptor in the infection is localized with their expression highest on the stromal side of the syncytium away from the maternal blood being the infection an event rare15. To describe the histopathological changes provoqued by the coronavirus on the structure of the placental villi is our proposal.
CASE REPORT
Pacient of 29 years old, with 38 weeks of pregnancy, who had Covid-19 at 36 weeks and whose placenta presented zones of hemorrhage, hematomes, infarcts, sclerosis of vessels and calcifications. With fever during a day, anosmy and hipogeusy and was recupered, live newborn, of 3050gr and puerpery normal.
The infected woman pregnancy had knowledge of informed consent and approval by the ethical committee of the hospitalary institution for the realization of this investigation according to the Helsinki declaration.
Five zones of the maternal-decidual region were taken: Four peripheric and one central. Small pieces of placenta were fixed in 10% formaldehyde, dehydrated in absolute alcohol, included in parafine, cuted in the microtome at 4-5um, desparafined in xilol, colored with H&E stain, deshidrated and aclared with Canada Balsam.
Regions of interest were photographied with a light photomicroscope MC63A Zeiss Clinic Standart (Carl Zeiss, Oberkochen, West Germany), observed at 400x, compared with normals control samples of patients without Covid-19 or another disease and prepared with similar procedures.
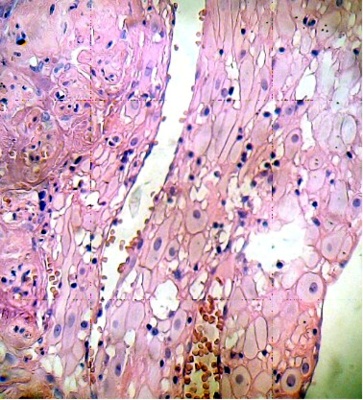
Cells of the placental decidual region are invaded by mononuclear cells. This region contains areas of cellular necrosis (Fig. 1). Some placental villi were infiltrated by numerous mononuclear cells in the intervillous space. The cytoarchitecture of the villi exhibits death cells with picnotic nucleus and their internal organization has been lost (Fig. 2).
|
|
Numerous stem villi have lost their endothelium and edematous region can to be observed in the stroma which is not noted in control sample (Fig. 3).
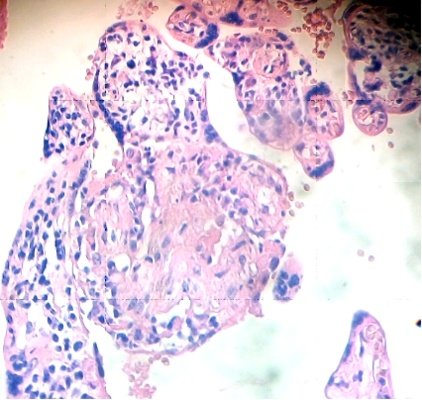
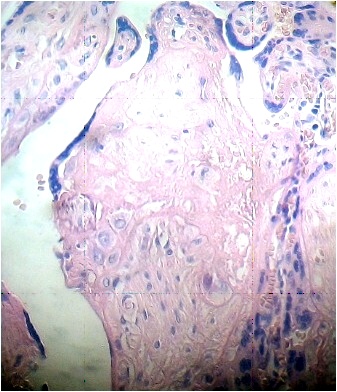
Under the syncytium some hypertrophied cytotrophoblasts are fusioned and have originated a small syncytium or multinucleated giant cell (Fig. 4). Any stem villi are observed degenerated with lost of syncytium, fibrotic stromal region in their inferior zone and deposition of fibrinoid that contains death cells (Fig. 5). Others are seen destroyed in their stromal region with calcium deposition (Fig. 6).
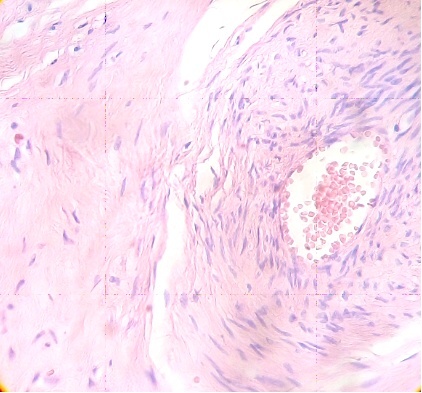
Fig.3. Vessel of stem villi with stromal oedema and endothelial damage. H&E.400x |
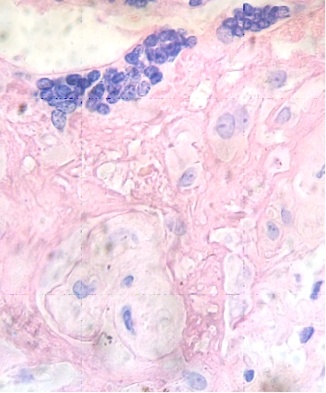
Fig.4. Hipertrophied cytotrophoblasts in fusion under the syncytium. H&E. 400x |
|
|
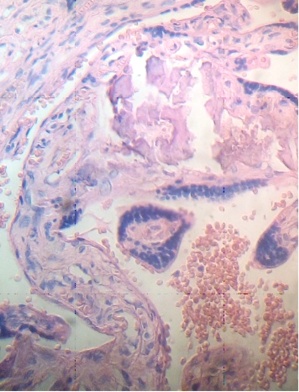
These figures were not seen in normal placenta. In the intervillous space can to be observed several villi destroyed (Fig. 7) and with deposition of calcium (Fig. 8). Rest of stem villi were seen with frecuency between damaged villi (Fig. 9). In regions of infarct numerous syncytial knots are noted (Fig. 10). Mature intermediate villi contains in the syncytium a double file of nuclei (Fig. 11)and extensive zones of syncytium contain numerous nuclei in others placental villi (Fig. 12).
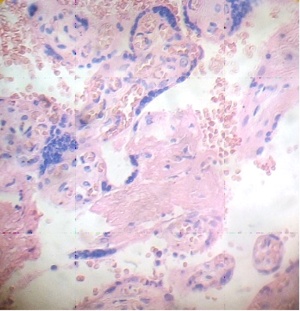
Fig.7. Villi are seen as exploited in the intervillous space. H&E. 400x |
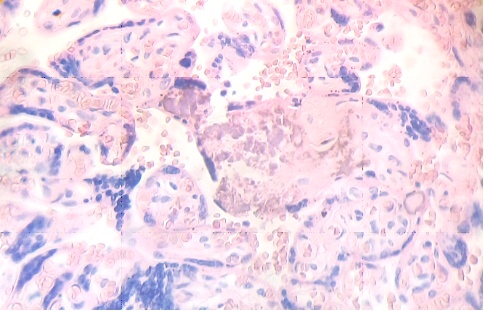
Fig.8. Numerous villi are seen fractured with deposition of calcium. H&E. 400xx |
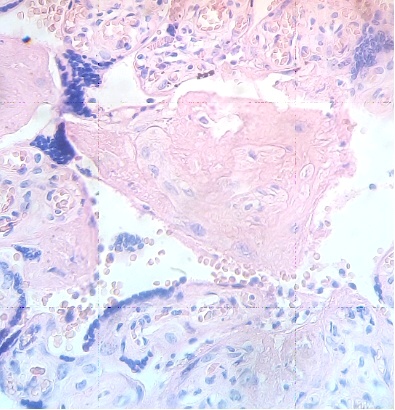
Fig.9. Rest of stem villi. H&E. 400x |
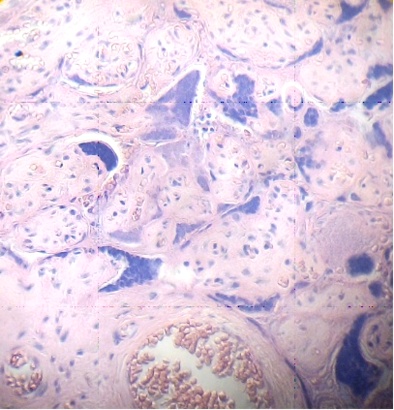
Fig.10. Region of infarct with numerous nuclei in syncytium and syncytial knots. H&E. 400x |
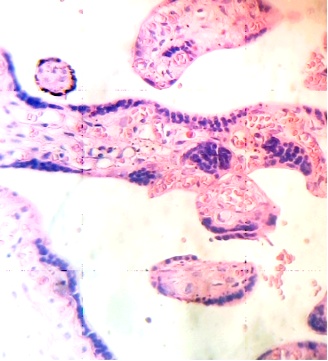
Fig.11. Double file of nuclei in syncytium of mature intermediate villi. H&E. 400x |
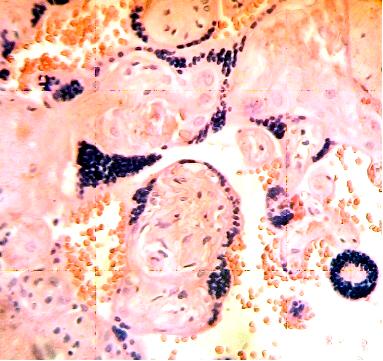
Fig.12. Extensive zones of syncytium contain numerous nuclei. H&E. 400x |
DISCUSSION:
The presence of villitis here described is evidence of a possible vertical transmission which could to spread the virus to the fetal bloodstream and to affect to the fetus 2. Papain-like protease 2 (PL2pro) and the 3C protease (3CLpro) as viral proteins present in the coronavirus could to be contributing with the interruption of membranes and cytoplasmic lysis in the cells of the placental villi provoquing their disorganization16.
This villitis altought very scanty, is the microscopic finding of inflammation of the chorionic villi that is the histologic hallmark of many maternal hematogenous infections that are transmitted through the placenta to the fetus which has been identified. Their scarcity could to be correlated with the negligible presence of ACE2 and TMPRSS2 described by Pique et al17.
By other hand, the vaculitis of stem villi, in these cases of placental insufficiency, can to induce in the ramifications of the placental tree events of thrombosis and subsecuent fetal vascular malperfusion, extensive fetal thrombotic vasculopathy, hypoxia and complications in the IUGR11, 14.
The betacoronavirus have a glycoprotein, the HE protein, with capability of haemagglutinating and binding to erythrocytes, an esterase activity, correlated with events of thrombosis16.
Hypertrophy cellular also has been seen in type II neumocytes10 and cell fusion was provoqued by Chikungunya virus to produce an enlarged and multinucleated cell18.
Death of the syncytium and stromal region with numerous death cells could to be provoqued by the non-structural protein 10 (nsp10), protein viral of the SARS coronavirus which interact with the cellular oxido-reductase system causing an extensive cytopathic effect since this molecule interrumpts the physiological function of mitochondria and cause severe damage to the cells19.
Pathological study suggest that there are not morphological changes related to infection in three placentas and no evidence for intrauterine vertical transmission16. Similar results have been found by He et al20. Howeber, Patané et al using advanced cell diagnostic with a ProbeV-nCov2019-S and automated equipment, visualizing the virus directly, has found the possibility of vertical transmission21.
But the majority of the literature has reported healthy neonates born to mothers with Covid-19 and the most frecuently reported pathological findings are the fibrosis, maternal vascular malperfusion, intervillous thrombi and increased wall of vessels in the chorionic plate22.
Pathological examinations have demonstrated that syncytium are often infected with SARS-CoV2, but fetuses are not always infected23. Maternal vascular malperfusion, injured maternal vessels, intervillous thrombi and villous edema, may reflect a systematic inflammatory or hypercoagulable state influencing adverse perinatal results during second and third trimester of pregnancy22. Although during the first trimester does not seem to predispose to early pregnancy loss, having a favorable maternal course 23.
Fibroblastic proliferation in fibrotic pneumonia has been observed in coronavirus infection of late stage. The incremented observation of nucleus here reported in the syncytium or syncytial knots could to be induced by these viruses24. The cytokine storm induced by SARS-CoV2 has provoqued this attack against the villous tree and can result in increased morbidity and mortality among pregnant women with the potential to adversely affect the developing fetus and neonate25.
In a pregnant woman with mild Covid-19 disease, with maternal vascular bad perfusion and fetal vascular good perfusion, the formation of microthrombi, accelerated villous maturation, infarction, intervillous thrombi, extravillous trophoblastic lesions, subchorionic necrosis, villous sclerosis and vascular kariorrhexis have been found in the placenta26.
Recenly Schwartz and Moretti have found that placentas from infected maternal-neonatal dyads are characterized by the finding of mononuclear cell inflammation of the intervillous space, termed chronic histiocytic intervillosites and that together with syncytial necrosis, in co-occurrence, appears to be a risk factor for maternal -fetal viral transmission27. Our samples have not this rare event possiblely by early treatment of the patient.
The presence of SARS-CoV2 has to be confirmed by placental sections, amniotic fluid or cord blood in order to investigate whether the placenta is infected and of this manner using transmission electron microscopy single virions were detected in the syncytium and stromal fibroblasts of a woman with severe Covid-1928
An early treatment has been indicated for Covid-19 with azithromycin and hidroxychloroquine29 since azithromycin inhibits SARS-CoV2 in vitro30.
Autophagy inhibitors as chloroquine, hidroxichloroquine, mefloquine, clomipramine, and others have suppressed the viral attack in culture of Vero-E6 cells inhibiting release of the viral genome and reducing the cytopathic effect. A viable target pathway for Covid-19 drug discovery according to a non-peer-reviewed pre print31.
In conclusion, Coronavirus have provoqued a strong attack to the placental villi disorganizing their structure which indicate that the placenta is not in their best condition for the interchange of gases and nutrients which could affect notably the fetal growth.
REFERENCES
-
1. MacLachlan NJ, Dubovi EJ . Coronaviridae. Fenner's Veterinary Virology 2017;435-461.
2. Schwartz DA, Dhaliwal A. Infections in pregnancy with Covid-19 and others respiratory RNA viruses diseases are rarely, if fever, transmitted to the fetus:experiences with coronavirus, HPIV,
hMPV, RSV and influenza. Arch Pathol Lab Med 2020;144 (8):920-928.
3. Kotlyar AM, Grechukhina O, Chen A, Popkhadze S, Grimshaw A et al. Vertical transmission of coronavirus disease 2019:a systematic review and meta-analysis. Am J Obstet Gynecol 2020;Jul 31:049.
4. Zamamiyan M, Ebadi A, Aghajanpoor SM, Rahmani Z, Haghshenas M et al. Preterm delivery in pregnant woman with critical Covid-19 pneumonia and vertical transmission. Prenatal diagnosis. Prenat Diagn 2020;Apr 17:10. 1002/pd. 5713.
5. Bonilla A DK, DhamaK, Rodriguez MAJ. Revisiting the one health approach in the contex of Covid-19:a look into the ecology of this emerging disease. Adv Anim Vet Sci 2020;8:234-237.
6. Rodriguez MAJ, Cardona OJA, Gutierrez OE, Villamizar PR, Holguin RY et al. Clinical laboratory and imaging features of Covid-19:A systematic review and meta-analysis. Travel Med Infect Dis 2020, 34:101623.
7. Baergen RN, Heller DS. Placental pathology in Covid-19 positive mothers. Preliminary findings. Pediat Dev Pathol 2020;23 (3):177-180.
8. Sharps MC, Hayes DJL, Lee S, Zou Z, Brady CH et al. A structured review of placental morphology and histopathological lesions associated with SARS-CoV2 infection. Placenta 2020; 101:13-29.
9. Zhu N, Wang W, Liu Z, Liang CH, Wang Wen et al. Morphogenesis and cytopathic effect of SARS-CoV2 infection in human airway epithelial cells. Nat Commun 2020;11:3910.
10. Tian S, Xiong Y, Liu H, Niu L, Guo J et al. Pathological study of 2019 novel coronavirus disease (Covid-19) through post mortem core biopsies. Mod Path 2020;33 (6):1007-1014.
11. Belen AFB, Sarialioglu F. Pulmonar intravascular coagulation in Covid-19:possible pathogenesis and recommendations on anticoagulant/thrombolytic therapy. J Thromb Thrombolysis 2020;50 (2):278-280.
12. Fachetti F, Bugatti M, Drera E, Tripodo C, Sartori E et al. Sars -CoV2 vertical transmission with adverse effects on the newborn revealed through integrated immunohistochemical, electron microscopy and molecular analysis of placenta. EBio Medicine 2020;59:102951.
13. Xu Z, Shi L, Wang Y, Zhang J, Huang L et al. Pathological findings of Covid-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8 (4):420-422.
14. Schwartz DA, Graham AL. Potential maternal and infant outcomes from coronavirus 2019-nCov (SARS-CoV2) infecting pregnant woman:Lessons from SARS, MERS and other human coronavirus. Viruses 2020;12 (2):194.
15. Hecht J, Quade B, Deshpande V, Mino KM, Ting DT et al. SARS-CoV2 can infect the placenta and is not associated with specific placental histopathology:a series of 19 placentas from Covid-19 positive mothers. Mod Pathol 2020; 2:1-12.
16. Chen S, Huang B, Luo DJ, Yang F, Zhao Y et al. Pregnancy with new coronavirus infection:Clinical characteristics and placental pathological analysis of three cases. Zhonghua Bing Li Xue Za Zhi 2020;49 (5):418-423
17. Pique RR, Romero R, Tarca A, Luca F, Xu Y et al. Does the human placenta express the canonical cell entry mediators for SARS-CoV2?. eLife 2020;9:e58716.
18. Castejon SOC. The placenta in a case of pregnant woman infected by Chikungunya virus. J Virol Retrovirol 2016;2 (1):1-4.
19. Li Q, Wang L, Dong Ch, Che Y, Jean L et al. The interaction of the SARS coronavirus non-structural protein 10 with the cellular oxido-reductase system causes an extensive cytopathic effect. J Clin Virol 2005; 34 (2):133-139.
20. He M, Skaria P, Krentz K, Chen L, Hagemann LS et al. Histopathology of third trimester placenta from SARS-CoV2 positive woman. Fetal Pediatr Pathol 2020;Oct 12:1-10
21. Patané L, Morotti D, Rosaria GM, Sigismondi C, Giovanna PM et al. Vertical transmission of coronavirus disease 2019:severe acute respiratory syndrome coronavirus 2 RNA on the fetal side of the placenta in pregnancies with coronavirus disease 2019-positive mothers and neonate at birth. Am J Obstet Gynecol MFM;2020;2 (3):100145.
22. Taglauer E, Benarroch Y, Rop K, Barnett E, Sabharwal V et al. Consistent localization of SARS-CoV2 spike glycoprotein and ACE2 over TMPRSS2 predominance in placental villi of 15 Covid-19 positive maternal-fetal dyads. Placenta 2020;100:69-74.
23. Komine AS, Fakada K, Hayakawa S. Placental barrier against Covid-19. Placenta 2020;99:45-49.
24. von der Thusen, van der Eerden M. Histopahtology and genetic susceptibility in Covid-19 pneumonia. Eur J Clin Invest 2020;50:e13259.
25. Verma S, Carter EB, Misorekar IU. SARS-CoV2 and pregnancy:An invisible enemy? Am J Reprod Immunol 2020;1:e13308.
26. Hsu AL, Guan M, Johannesen E, Stephens AJ, Khaleel N et al. Placental SARS-CoV2 in a pregnant woman with mild Covid-19 disease. J Med Virol 2020; jmv. 26386.
27. Schwartz DA, Morotti D. Placental pathology of Covid-19 with and without fetal and neonatal infection:Trophoblast necrosis and chronic histiocytic intervillositis as risk factors for transplacental transmission of SARS-CoV2. Viruses 2020;12 (11):1308.
28. Algarroba GN, Rekawek P, Vahanian SA, Khullar P, Palaia T, Peltier MR, Chavez MR, Vintzileos AM. Visualization of SARS-CoV2 virus invading the human placenta using electron microscopy. Am J Obstet Gynecol. 2020;223(2):275-278.
29. Millon M, Christophe L J, Gautret P, Calson P, Edouard FP et al. Early treatment of Covid-19 patients with hidroxichloroquine and azithromycin : A retrospective analysis of 1061 cases in Marseille, France. Trav Med Infect Dis 2020;35:101738.
30. Andreani J, Bideau M, Duflot I, Jardot P, Rolland C et al. In vitro testing of combined hidroxichloroquine and azithromycin on SARS-CoV2 shows synergistic effect. Microb Pathog 2020;145:104228.
31. Gorshkov K, Chen CZ, Bostwick R, Rasmussen L, Xu M et al. The SARS-CoV2 cytopathic effect is blocked with autophagy modulators. bioRxiv 2020;May28;Preprint. doi:10. 1101/2020. 05. 16. 091 528.
CORRESPONDENCE:
Prof. Olivar Clemente Castejon Sandoval
Director of the Center for Research and Analysis Assistancel Teaching of the Nucleus Aragua (CIADANA).
Laboratory of Electron Microscopy.
Faculty of Health Sciences,
University of Carabobo.
Aragua State.
Maracay, Venezuela.
Email: olivar. ciadanauc @ gmail. com